Fig. 5.
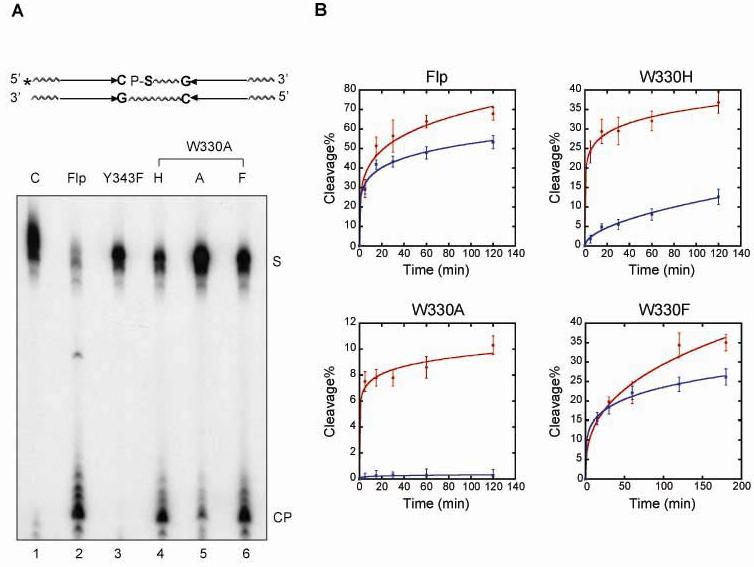
Strand cleavage by the Trp-330 Flp mutants in a substrate containing a 5'-thiolate linkage at the cleavage position. In the 5' end-labeled cleavage substrate, the scissile phosphate on the top strand was linked via a 5'-thiolate bridge to the neighboring nucleotide to the 3' side. A. Reactions from 30 min incubations at 30°C were processed, and fractionated by denaturing polyacrylamide gel electrophoresis. The substrate and product bands are denoted by ‘S’ and ‘CP’, respectively. B. Time course cleavage reactions were carried out using a standard phosphate containing substrate (blue lines) or a 5'-thiolate containing substrate (red lines). Following separation of ‘S’ and ‘CP’ by denaturing polyacrylamide electrophoresis, they were quantitated using a phosphorimager. The estimated cleavage yields for wild type Flp and the mutants were plotted as a function of time. The values were obtained from four separate assays.
