Fig. 7.
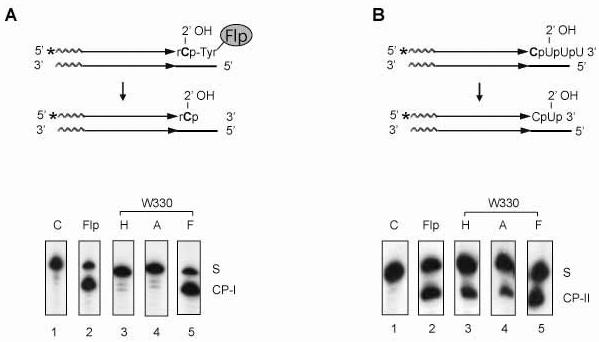
The activities of the Trp-330 mutants of Flp in type I and type II Flp RNA cleavage reactions. A. in the type I RNA cleaving activity of Flp, the vicinal 2'-hydroxyl attacks the phosphate group covalently linked to Flp via Tyr-343. The cleavage product ‘CP-I’, containing a 3'-phopshate end, was separated from the substrate ‘S’ by electrophoresis in a denaturing polyacrylamide gel. B. In the type II RNA cleaving activity of Flp, the vicinal 2'-hydroxyl group directly attacks the target phosphodiester bond. The cleavage product ‘CP-II’ (also with a 3'-phosphate end) was separated from the substrate ‘S’ as under A. The data shown are for 30 min reactions carried out at 30°C.
