Abstract
Recent evidence shows that the DNA repair protein Ku is expressed on the surface of a subset of cells, where it contributes to adhesion and invasion processes, and also to viral or bacterial entry into target cells. Here, we show that Ku was expressed on the cell surface during activation of human monocytes and that its expression was independent of the conventional endoplasmic reticulum (ER)/Golgi secretory pathway. Ku inhibition, by blocking antibodies, decreases the migration of monocytes on fibronectin and laminin. On activation, nuclear Ku seems to move to the periphery of the cell and it shows a punctuate staining in the cytoplasm. The cytoplasmic distribution of Ku was shown to be unaltered by brefeldin A. Protease protection experiments show that Ku is contained within vesicles in activated monocytes. These data support a new role for Ku in the migration of monocytes into tissues, which depends on a tightly regulated pathway of intracellular redistribution.
Keywords: monocytes, non-classical secretion, Ku heterodimer, DNA repair, moonlighting, migration
Introduction
Recent data indicate that the Ku heterodimer is a moonlighting protein. ‘Moonlighting'—a term coined by Jeffery (1999)—refers to the ability of certain proteins to fulfil more than one, apparently unrelated, function. In the nucleus, the Ku protein—a heterodimer of two tightly associated subunits called Ku70 and Ku80—has an important role in nonhomologous end joining repair, a process responsible for repairing the majority of DNA double-strand breaks in mammalian cells (for a review, see Hefferin & Tomkinson, 2005). In addition to its intranuclear role, we and others have shown that Ku is also expressed on the surface of various untransformed normal—that is, macrophages, endothelial cells, erythroid progenitors—and tumour cells, as well as tumour cell lines (Muller et al, 2005). The membrane-associated form of Ku mediates parvovirus B19 infection of erythroid cells (Munakata et al, 2005) and is involved in the internalization of Rickettsia conorii (Martinez et al, 2005). A role for Ku in cellular adhesion (Teoh et al, 1998; Lynch et al, 2001), cell–matrix attachment (Monferran et al, 2004a) and proteolytic process through matrix metalloproteinase (MMP) 9 interaction (Monferran et al, 2004b) has also been shown. According to these coordinated functions, cell-surface Ku might be involved in invasion processes observed not only for cancer cells, but also for normal cells such as monocytes during recruitment to tissues, a crucial event during inflammatory responses. Previously we have shown that Ku is expressed on the cell surface of monocyte-derived macrophages, but regulation of its expression and function in monocytes is unknown. Here, we show that cell-surface Ku accumulates rapidly after induction of monocyte differentiation by cytokines or lipopolysaccharide and contributes to subendothelial migration. The possibility of exporting cytosolic or nuclear proteins such as Ku that lack the canonical amino-terminal secretion independently of the endoplasmic reticulum (ER)/Golgi apparatus is widespread, but is poorly characterized at the molecular level (Nickel, 2003). Interestingly, the expression of these proteins outside the cells seems to be biologically significant and frequently contributes, as for Ku, to their ‘moonlighting' abilities as a consequence of changes in cellular localization (for a review, see Jeffery, 1999).
Results And Discussion
Ku is expressed on the cell surface of activated monocytes
By using flow cytometry experiments, in which fresh living cells were stained, recently isolated monocytes from the blood of healthy donors were shown to be negative for Ku cell-surface expression (Fig 1A). We have described previously that Ku is expressed on the cell surface of monocyte-derived macrophages (Monferran et al, 2004b), indicating that Ku cell-surface expression depends on monocyte differentiation and activation. According to this hypothesis, overnight exposure of these cells to monocyte colony-stimulating factor (M-CSF) increases the amount of both Ku subunits detected at the surface of activated cells (Fig 1A; supplementary Fig S1 online). The expression of cell-surface Ku in activated monocytes was also observed in response to other stimuli such as lipopolysaccharide (data not shown). Under these conditions, we verified further that the expression of other activation markers was increased as described (Fig 1A; Murtaugh et al, 1984; Prieto et al, 1994). By contrast, negative staining of the nuclear protein, cyclin-dependent kinase activating kinase (CAK), was observed at the cell surface in both resting and M-CSF-treated monocytes (Fig 1A). Our results show that activation of monocytes is associated with cell-surface expression of Ku as observed in all the tested samples with little variation among donors (supplementary Fig S2 online). The level of cell-surface expression of Ku is lower than the other activation markers, such as integrin β1 or CD44, but close to that of tissue transglutaminase (tTG). As for tTG (Murtaugh et al, 1984), the culture of monocytes for up to 7 days (which corresponds to the conversion of monocytes into macrophages) was associated with a progressive and marked increase in the amount of Ku70 detected at the cell surface, with a first rapid phase of accumulation within 18 h after stimulation (supplementary Fig S3 online). Once activated, and after endothelial transmigration, monocytes must traverse tissue barriers (subendothelial basement membrane and interstitial matrix) composed of distinct structural proteins to reach inflammatory focus. The subendothelial basement membrane is a dense and continuous meshwork of proteins, which include laminin, type IV collagen and proteoglycans; the interstitial matrix is rich in fibronectin. In opposition to trans-endothelial migration, little is known about the subsequent migration of monocytes across these barriers. We have shown previously that Ku mediates fibroblasts adhesion on fibronectin, and presents a structural relationship with integrin I and the A1 and A3 domains of von Willebrand factor, domains known to mediate fibronectin binding (Monferran et al, 2004a). The role of Ku in the interstitial migration of human monocytes on fibronectin was then evaluated. As shown in Fig 1B, inhibitory monoclonal antibodies directed against either the Ku70 or Ku80 subunit significantly inhibited migration of monocytes on fibronectin towards the chemokine monocyte chemotactic protein 1 (MCP1) by about 40–50%, in contrast to the isotype-matched control. When increasing concentrations of MCP1 were present in the upper wells, we observed a decrease in the ability of Ku antibodies to inhibit migration, indicating that Ku might be involved in chemotaxis rather than chemokinesis (supplementary Fig S4 online). Incubation with Ku70 and β1- and β3-antibodies showed an additional inhibitory effect, suggesting that these antibodies share different targets on the cell surface (data not shown). Similar migration experiments were carried out using other substrates. Treatment with blocking antibodies directed against Ku70 or Ku80 did not change the migration of activated monocytes on collagen I and IV, but significantly reduced migration on laminin (Fig 1C). Altogether, these data show that Ku is detected at the surface of activated monocytes and contributes to the migration of these cells through fibronectin and laminin matrices, highlighting the importance of the membrane-associated form of Ku in a physiological situation. Interestingly, the results obtained in the present study suggest that these effects on migration might be independent of Ku adhesive functions, as we have previously shown that there was no increase in cell adhesion on laminin matrices in cells that expressed cell-surface Ku (Monferran et al, 2004a).
Figure 1.
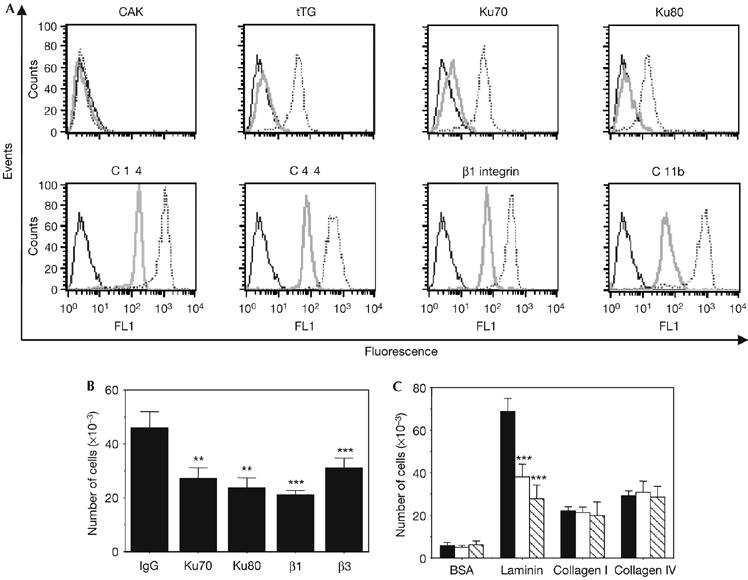
Monocyte differentiation is associated with expression of Ku on the cell surface, which contributes to the regulation of the cell migration on fibronectin. (A) Cell-surface expression of the indicated antigens was analysed by flow cytometry in resting (grey) or differentiated (18 h; dashed) monocytes. The results obtained for isotype-matched controls in differentiated monocytes are shown (black) and were identical between resting and activated monocytes. (B) Monocytes were treated overnight with 10 ng/ml of M-CSF and then placed into the upper chambers of Transwells, undercoated with fibronectin. The cells were pre-incubated for 1 h with the indicated antibodies before being added to fibronectin-coated wells. The means of three separate experiments carried out in duplicate are shown. **Statistically significant by Student's t-test, P<0.01 relative to control; *** statistically significant by Student's t-test, P<0.001 relative to control. (C) Similar experiments were carried out with inserts undercoated with the indicated substrates. Cells were pre-incubated with control Ig (black), Ku70 (white) or Ku80 (hatched) antibodies. The means of three separate experiments carried out in duplicate are shown. Statistically significant by Student's t-test, ***P<0.001 relative to control. BSA, bovine serum albumin; CAK, cyclin dependent kinase activating kinase; FL1-H, fluorescence pulse 1-height; Ig, immunoglobulin; M-CSF, monocyte colony-stimulating factor; tTG, tissue transglutaminase.
Ku export is resistant to ER/Golgi inhibitors
We have found that the first phase of upregulation of the membrane-associated form of Ku occurs in the first 18 h after stimulation (Fig 1B). As shown in Fig 2A,B, this upregulation was independent of de novo protein synthesis. No marked change in the total cellular levels of Ku was observed during monocytes activation (Fig 2A), and exposure of treated monocytes to cycloheximide had no effect on the Ku detected at the cell surface, in contrast to CD44 (Fig 2B). These results show that the amount of Ku proteins on the cell surface, but not the total amount of Ku proteins, is increased after activation, and that the activation signals sent by M-CSF, leading to membrane translocation of Ku, do not require protein synthesis. Ku cell-surface expression has been reported to increase in response to external stimuli such as hypoxia in monocytic cells (Lynch et al, 2001), or on exposure of multiple myeloma cells to CD40L (Teoh et al, 1998). In both cases, upregulation of the expression of the membrane-associated Ku was the result of a translocation of an intracellular pool to the cell surface, but the mechanism of shuttling to the cell surface remains unknown. Both Ku proteins lack conventional signal peptides and ER/Golgi-dependent post-translational modification, such as N-glycosylation (Ginis et al, 1995); therefore, we postulated that Ku might be secreted by non-classical export. So far, more than 20 proteins devoid of secretory signal sequence are released by cells by a pathway independent of the ER/Golgi complex (Rubartelli et al, 1990; Nickel, 2003). Accordingly, the secretion of these proteins is resistant to brefeldin A (BFA) or monensin, two inhibitors of the conventional ER/Golgi secretory pathway (Nickel, 2003). In contrast to CD44 and integrin β1, cell-surface expression of Ku was unaffected by BFA (Fig 2C, upper panel) or by monensin (Fig 2C, lower panel), suggesting that the membrane-associated form of Ku follows an unconventional pathway for its secretion.
Figure 2.
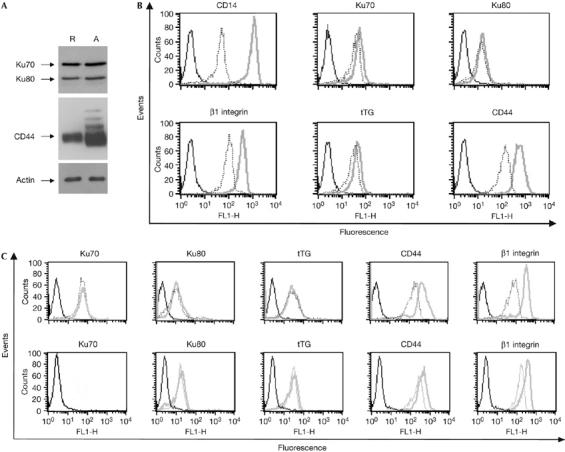
Ku expression on the cell surface is resistant to inhibitors of the endoplasmic reticulum/Golgi secretory pathway. (A) Whole-cell extracts of resting (R) or activated (A) monocytes were analysed by western blot using the indicated antibodies. (B) Monocytes were incubated in complete medium containing M-CSF in the presence or absence of cycloheximide for 18 h. Cell-surface expression of the indicated antigens in activated monocytes treated (dashed) or not treated (grey) with cycloheximide was analysed by flow cytometry. (C) Monocytes were grown for 18 h in complete medium containing M-CSF in the presence or absence of 5 μg/ml BFA (upper panel) or 20 μM monensin (lower panel). After treatment, cell-surface expression of the indicated antigens was analysed by flow cytometry in treated (dashed) or untreated (grey) monocytes. BFA, brefeldin A; FL1-H, fluorescence pulse 1-height; M-CSF, monocyte colony-stimulating factor; tTG, tissue transglutaminase.
Ku relocalizes to the cytoplasm in activated monocytes
To investigate further how this nuclear protein reaches the extracellular space, the subcellular distribution of Ku during monocyte activation was investigated by immunofluorescence. As shown in Fig 3, resting monocytes show a strong staining for Ku80 that is restricted to the nucleus. One hour after exposure to M-CSF, nuclear Ku80 seems to move to the periphery of the cell, where it shows a punctuate staining in the cytoplasm. The expression of Ku80 in the cytoplasm is still observed 18 h after activation with an even more characteristic punctuated pattern (Fig 3A). At higher magnification in areas close to the nucleus, most of these aggregates seemed to be blebbing (see inset). A similar pattern of Ku80 subcellular redistribution was also observed in lipopolysaccharide (LPS)-activated monocytes (Fig 3B) and for the Ku70 subunit (Fig 3C). Thus, the pattern of Ku expression in the cytoplasm might indicate that Ku is contained within a vesicular compartment.
Figure 3.
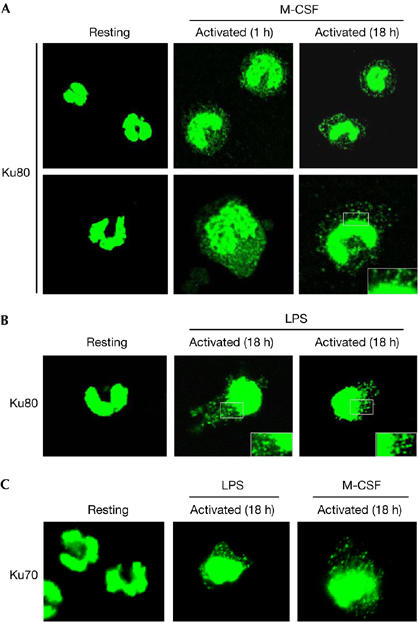
Ku is translocated from the nucleus to the periphery in monocytes treated with monocyte colony-stimulating factor or lipopolysaccharide. Freshly isolated monocytes or monocytes incubated in complete medium containing M-CSF (A) or LPS (B) for the indicated time period were fixed, stained with the Ku80 (S10B1) antibody and analysed by confocal microscopy. Insets show twofold magnified views of peripheral nuclear regions. (C) Similar experiments were carried out with the Ku70 (S5C1) antibody in resting or activated monocytes as indicated. LPS, lipopolysaccharide; M-CSF, monocyte colony-stimulating factor.
Ku is contained within vesicles in activated monocytes
At least four distinct types of nonclassical export have been described. One mechanism of export, described for interleukin 1β (Andrei et al, 1999) and high mobility group B1 (HMGB1; Gardella et al, 2002), involves import into intracellular vesicles, which are probably endolysosomal related vesicles (for a review, see Nickel, 2003). To investigate the nature of Ku-positive spots, a particulate fraction (P50) was isolated from post-nuclear supernatants of resting or M-CSF-treated monocytes (Rubartelli et al, 1990; Andrei et al, 1999, Gardella et al, 2002) and western blot analysis was carried out. As described previously (Rubartelli et al, 1990; Andrei et al, 1999, Gardella et al, 2002), the mature, most abundant 31 kDa form of cathepsin D was present in this dense vesicular compartment and equally represented between resting and activated monocytes (Fig 4A). Similarly to interleukin 1β (Andrei et al, 1999; Gardella et al, 2002), Ku70 and Ku80 are present in the P50 fraction of M-CSF-treated, but not resting, monocytes (Fig 4A). Both Ku subunits were protease-protected, but the protection against proteinase K digestion was abolished when vesicular membranes were solubilized using detergent, indicating that the protection was due to the encapsulation by membranes. Similar results were obtained with interleukin 1β, although a minor proteinase K-resistant interleukin 1β band (as the mature form of cathepsin D) was present in the presence of Triton X-100, consistent with previous observations (Andrei et al, 1999; Gardella et al, 2002). To characterize further the intracellular distribution of Ku in activated monocytes, the effect of BFA treatment was investigated. Treatment with BFA causes rapid disassembly of protein transportation between the ER and the Golgi apparatus, and redistributes this protein into the ER (Lippincott-Schwartz et al, 1989; Sciaky et al, 1997). It also causes membrane proteins resident in the trans-Golgi network (TGN) to disassemble and to redistribute to the microtubule organizing centre (Reaves & Banting, 1992). Therefore, BFA can be used to determine whether a protein of interest is associated with the Golgi complex or the trans-Golgi network. As shown in Fig 4B, BFA treatment had no effect on the intracytoplasmic distribution of Ku. By contrast, treatment of activated monocytes with BFA led to a complete loss of Golgi structure, as assessed by giantin relocalization. These results indicate that Ku was not found within compartments of the classical secretory pathway. Ku is found in intracellular vesicles in activated monocytes, as judged by protease protection experiments. These structures seem to be unrelated to the ER/Golgi system as Ku export is not inhibited by monensin or BFA and its intracellular distribution is insensitive to BFA. As no particulate Ku is detectable in resting monocytes, which express Ku in the nucleus but do not export it to the extracellular space, these vesicular elements are probably part of the protein secretory pathway. The nature of these vesicular elements remains to be established. Interestingly, the cytoplasmic distribution of Ku and its extracellular expression were insensitive to the inhibitors of ABC transporters (glibenclamide and methylamine; data not shown) in opposition to interleukin 1β (Andrei et al, 1999). This suggests that different mechanisms of import into vesicles and export might be present among the proteins secreted by the vesicle-related non-conventional pathway. We then investigated whether the expression of Ku on the cell surface might be dependent on an intact actin cytoskeleton and/or microtubules. Ku cell-surface expression was abolished when actin was disrupted by cytochalasin D treatment (see supplementary Fig S5 online); by contrast, nocodazol treatment has no marked effect (data not shown). Recent data suggest that actin microfilaments are involved not only in several aspects of vesicle transport including organelle movement, but also in biogenesis (Taunton, 2001). The role of actin cytoskeleton in the mechanism of non-classical, vesicle-mediated secretion has not yet been explored and our results indicate its involvement in this vesicular transport. It would be of great interest to determine whether the actin-dependent translocation of the proteins to the cell surface is a common feature of this pathway.
Figure 4.
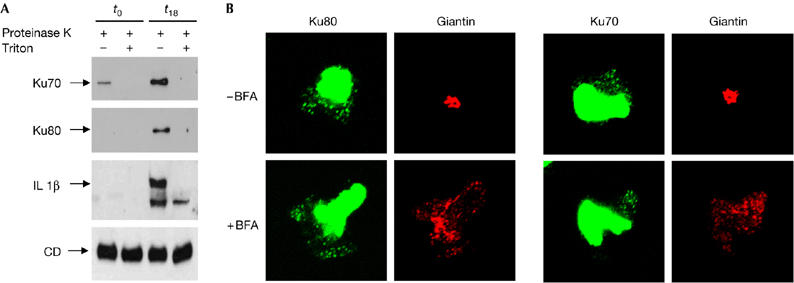
Ku is incorporated in vesicles in treated monocytes. (A) A 25 μg portion of the particulate post-nuclear fraction from resting (t0) or activated (t18) monocytes, treated with proteinase K alone or in the presence of Triton X-100, was analysed by western blotting using the indicated antibodies. (B) Monocytes grown on coverslips in complete medium with M-CSF were treated with or without BFA for 90 min (5 μg/ml). Monocytes were fixed and double stained with Ku80 and Giantin antibodies. Similar double staining was carried out with Ku70 and Giantin antibodies. BFA, brefeldin A; CD, cathepsin D; IL 1β, interleukin 1β; M-CSF, monocyte colony-stimulating factor.
Ku is expressed at the surface of a limited number of cells (Muller et al, 2005) in contrast to its nuclear expression. Our data show that in monocytes, pro-inflammatory stimuli contribute to the export of Ku outside the cell in an active and tightly controlled manner. Our observations highlight a new role for cell-surface Ku in cell migration into interstitial matrices. Ku might upregulate cell motility, affecting migration mediated by cell-surface proteins other than β1- or β3-integrins. In fact, we have previously shown that Ku behaves like a peripheral, rather than an integral, membrane protein (Monferran et al, 2004b). Additional experiments carried out here show that Ku is not a glycosyl phosphatidyl inositol (GPI) anchor protein (see supplementary Fig S6 online) and is probably associated with the cell surface by an as yet uncharacterized integral membrane-anchoring protein. Thus, Ku might contribute to regulate migration through its association with cell-surface docking molecules. Alternatively, its ability to anchor MMP9 on the cell surface (Monferran et al, 2004b) might, in addition to upregulation of pericellular proteolysis, modulate the migratory state of cells as reported previously (Sanceau et al, 2003). Interestingly, we have recently shown that Ku is also expressed on the surface of primary acute leukaemia cells with the monocytic features that show extramedullary localization (J.P., V. De Mas, C. Demur, B.S. & C.M., unpublished data). These results indicate that leukaemia cells have illicitly acquired the biochemical machinery for transport as used by their mature counterpart and highlight the important role of Ku in monocyte function in both physiological and pathological conditions. There is now emerging evidence that a group of nuclear proteins, such as HMGB1 and now including Ku, are used outside the cell in a tightly coordinated manner to respond to tissue injury. The use of these moonlighting proteins can provide a means of coordinating cellular activities, and one of the future challenges will be to understand whether the cell coordinates the subcellular distribution of Ku in time and space and whether a crosstalk exists between these different localizations.
Methods
Cells. Human blood mononuclear cells were isolated from healthy donor blood by Ficoll density gradient centrifugation as described previously (Gardella et al, 2002). Monocytes were purified by immunomagnetic selection using the monocyte-negative isolation kit (Invitrogen, Cergy Pontoise, France) according to the manufacturer's instructions; resting monocytes were immediately processed for flow cytometric experiments. Monocytes were cultured in RPMI containing 10% FCS (fetal calf serum) for the indicated time periods in the presence of 10 ng/ml M-CSF or 1 μg/ml LPS (Sigma-Aldrich, Lyon, France).
Isolation of the P50 fraction. Subcellular fractionation was carried out as described previously (Gardella et al, 2002). Resting or M-CSF-treated monocytes (18 h) were homogenized in 250 mM sucrose, 5 mM EGTA and 20 mM HEPES–KOH (pH 7.2) in a Dounce homogenizer and centrifuged at 800 g for 10 min to remove nuclei, cell debris and unbroken cells. Post-nuclear supernatants were subjected to differential ultracentrifugation (50,000 g for 5 min) to obtain the P50 fraction. This pellet was treated with 0.1 mg/ml of proteinase K (Eurobio, Les Ullis, France) for 30 min at 4°C, in the absence or presence of 1% Triton X-100, followed by addition of 1 mM of phenylmethylsulfonyl fluoride for 10 min at 4°C to stop the reaction. Samples were then analysed by western blot.
Other methods. Further methods are described in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary information Fig S1
supplementary information Fig S2
supplementary information Fig S3
supplementary information Fig S4
supplementary information Fig S5
Supplementary information Fig S6
Supplementary Information
Acknowledgments
We thank S. Carreno for helpful comments on the manuscript. This work was supported by grants from ‘Electricité Gaz de France', the ‘Ligue Nationale Contre le Cancer (Equipe Labelisée)' and the ‘Commissariat à l'Energie Atomique (Corresponding Laboratory)'.
References
- Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A (1999) The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol Biol Cell 10: 1463–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3: 995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginis I, Mentzer SJ, Li X, Faller DV (1995) Characterization of a hypoxia-responsive adhesion molecule for leukocytes on human endothelial cells. J Immunol 155: 802–810 [PubMed] [Google Scholar]
- Hefferin ML, Tomkinson AE (2005) Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair 4: 639–648 [DOI] [PubMed] [Google Scholar]
- Jeffery CJ (1999) Moonlighting proteins. Trends Biochem Sci 24: 8–11 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56: 801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch EM, Moreland RB, Ginis I, Perrine SP, Faller DV (2001) Hypoxia-activated ligand HAL-1/13 is lupus autoantigen Ku80 and mediates lymphoid cell adhesion in vitro. Am J Physiol Cell Physiol 280: C897–C911 [DOI] [PubMed] [Google Scholar]
- Martinez JJ, Seveau S, Veiga E, Matsuyama S, Cossart P (2005) Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell 123: 1013–1023 [DOI] [PubMed] [Google Scholar]
- Monferran S, Muller C, Mourey L, Frit P, Salles B (2004a) The Membrane-associated form of the DNA repair protein Ku is involved in cell adhesion to fibronectin. J Mol Biol 337: 503–511 [DOI] [PubMed] [Google Scholar]
- Monferran S, Paupert J, Dauvillier S, Salles B, Muller C (2004b) The membrane form of the DNA repair protein Ku interacts at the cell surface with metalloproteinase 9. EMBO J 23: 3758–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Paupert J, Monferran S, Salles B (2005) The double life of the Ku protein: facing the DNA breaks and the extracellular environment. Cell Cycle 4: 438–441 [DOI] [PubMed] [Google Scholar]
- Munakata Y, Saito-Ito T, Kumura-Ishii K, Huang J, Kodera T, Ishii T, Hirabayashi Y, Koyanagi Y, Sasaki T (2005) Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood 106: 3449–3456 [DOI] [PubMed] [Google Scholar]
- Murtaugh MP, Arend WP, Davies PJ (1984) Induction of tissue transglutaminase in human peripheral blood monocytes. J Exp Med 159: 114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W (2003) The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 270: 2109–2119 [DOI] [PubMed] [Google Scholar]
- Prieto J, Eklund A, Patarroyo M (1994) Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol 156: 191–211 [DOI] [PubMed] [Google Scholar]
- Reaves B, Banting G (1992) Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol 116: 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R (1990) A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J 9: 1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanceau J, Truchet S, Bauvois B (2003) Matrix metalloproteinase-9 silencing by RNA interference triggers the migratory-adhesive switch in Ewing's sarcoma cells. J Biol Chem 278: 36537–36546 [DOI] [PubMed] [Google Scholar]
- Sciaky N, Presley J, Smith C, Zaal KJ, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J (1997) Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol 139: 1137–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J (2001) Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr Opin Cell Biol 13: 85–91 [DOI] [PubMed] [Google Scholar]
- Teoh G, Urashima M, Greenfield EA, Nguyen KA, Lee JF, Chauhan D, Ogata A, Treon SP, Anderson KC (1998) The 86-kD subunit of Ku autoantigen mediates homotypic and heterotypic adhesion of multiple myeloma cells. J Clin Invest 101: 1379–1388 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information Fig S1
supplementary information Fig S2
supplementary information Fig S3
supplementary information Fig S4
supplementary information Fig S5
Supplementary information Fig S6
Supplementary Information


