Recent research has opened new avenues for the evaluation of mitochondrial function; for example, mitochondria are no longer perceived as thread-like static entities within the cytosol, but instead are viewed as highly dynamic organelles that can change in shape and size, and are transported to strategic locations within the cell. Mitochondrial morphology, size and position within cells are maintained through a balance of fission and fusion events. Perturbation of the steady state between these opposing processes has been directly implicated in several human disorders (Chan, 2006). Although the list of genes for mitochondrial morphogenesis is rapidly increasing, dynamin-related protein 1 (Drp1)—a cytosolic dynamin GTPase—was among the first fission proteins to be discovered; however, the mechanism by which Drp1 function is regulated is poorly understood.
In this issue of EMBO reports, Cribbs & Strack identify a new mechanism by which second messengers—cAMP and calcium—modulate mitochondrial shape and function through the regulation of Drp1 phosphorylation. Cyclic-AMP-dependent protein kinase (PKA)-mediated phosphorylation of Drp1 at Ser 656 induces mitochondrial elongation and resistance to apoptotic stimuli, whereas dephosphorylation of Ser 656 by calcineurin promotes mitochondrial fragmentation and increases cell vulnerability to apoptosis. These studies provide a new mechanistic insight into the link between the mitochondrial fission machinery and cell death signalling.
Drp1 is recruited to the mitochondrial surface at potential fission sites (Ingerman et al, 2005; Okamoto & Shaw, 2005). The energy generated by GTP hydrolysis is believed to provide the mechanical force required to execute fission (Ingerman et al, 2005). Although gain- and loss-of-function studies of Drp1 correlate mitochondrial fission with apoptosis (Frank et al, 2001; Germain et al, 2005), there is no evidence to show that Drp1 alone, or mitochondrial fission by itself, can induce apoptosis. In addition, although Drp1 GTPase can be regulated by ubiquitination and sumoylation (Nakamura et al, 2006; Wasiak et al, 2007), there is little insight as to how Drp1 activation might be regulated during apoptosis signalling. In this issue, Cribbs & Strack have addressed some of these important questions and provide a mechanistic link for second messenger regulation of Drp1 GTPase activity and apoptosis signalling.
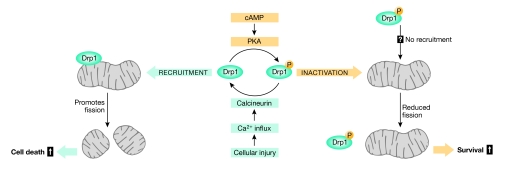
First, Cribbs & Strack show that PKA-mediated-phosphorylation of Drp1 at Ser 656 attenuates the GTPase activity of Drp1 and promotes cell survival, suggesting that cAMP might mediate survival partly through the inhibition of Drp1 (Fig 1). An independent study (Chang & Blackstone, 2007) corroborates these findings by showing that PKA-dependent phosphorylation of Drp1 within the GED domain at Ser 637 blocks Drp1 GTPase activity. Phosphomimetic substitution at Ser637Asp was also shown to block mitochondrial fission (Chang & Blackstone, 2007). The question of how phosphorylation at Ser 656 and Ser 637 might differ in regulating the GTPase activity remains to be resolved. One possibility is that a spatiotemporal relationship exists whereby phosphorylation at one site regulates modification of the second site. In addition, a third Drp1 phosphorylation site has been reported and is believed to be involved in breaking down the mitochondrial network during mitosis (Taguchi et al, 2007). It is likely that Drp1 phosphorylation at different sites might have different physiological consequences. This hypothesis is supported further by the recent finding that phosphorylation modulates substrate processing in a site-specific manner (Jahani-Asl et al, 2007a). The complexity imposed by phosphorylation on the three-dimensional structure and oligomerization of Drp1 protein requires further investigation.
Figure 1.
Missing links between mitochondrial fission and apoptosis. Cyclic-AMP-dependent protein kinase (PKA) phosphorylates dynamin-related protein 1 (Drp1) and induces mitochondrial elongation and resistance to apoptosis. Calcineurin dephosphorylates Drp1, promotes mitochondrial fragmentation and cell vulnerability to apoptosis. Whether phospho-Drp1 confers protection through inhibiting mitochondrial fission or through other regulatory signalling molecules remains to be discovered.
A link between mitochondrial fission and death signalling pathways converging on mitochondria has been a hotly debated topic for several reasons. Mitochondrial fission is required under normal physiological conditions to ensure biogenesis and to respond to changes in energy demands (Yaffe, 1999). Although mitochondrial fission has been shown to occur as an early event during cell death (Barsoum et al, 2006; Jagasia et al, 2005; Jahani-Asl et al, 2007b), and Bax/Bak-mediated Drp1-induced mitochondrial fission promotes cell death (Arnoult et al, 2005), it has also been shown that inhibiting the fission machinery does not prevent Bax/Bak-dependent apoptosis (Parone et al, 2006). In addition, Drp1-mediated fission of mitochondria has been reported to protect against cell death (Szabadkai et al, 2004). More importantly, although in vitro studies often focus on death pathways evoked by single inducers, the scenario in vivo is quite complex (Cheung et al, 2007). Previous findings show that recruitment of Drp1 at the scission sites occurs simultaneously with Ca2+ uptake by mitochondria (Breckenridge et al, 2003). In this issue, Cribbs & Strack show that calcium induces mitochondrial fission through Drp1 dephosphorylation (Fig 1). This finding has broad implications pertaining to several modes of cell death including death induced by staurosporine (a kinase inhibitor), etoposide (topoisomerase inhibitor), calcium ionophore A23187 and oxidative stress. The question of how Drp1 phosphorylation might protect against cell death remains open: one possibility is that phosphorylation might modulate Drp1 interaction with other regulatory proteins that assist in targeting Drp1 to mitochondria. Finally, Cribbs & Strack show that Drp1 phosphorylation protects against apoptotic insult despite the fact that a population of mitochondria exhibit ultrastructure abnormalities. This apparent inconsistency suggests that Drp1 might have other roles in addition to regulating mitochondrial fission.
In summary, these new studies suggest that components of the mitochondrial fission–fusion machinery are linked to cellular signalling pathways and identify a new mechanism by which second messengers might regulate mitochondrial structure and function. Future research towards identifying upstream and downstream regulators of the fission–fusion machineries might identify new approaches to modulate the onset of cell death that becomes deregulated in many human diseases.

Arezu Jahani-Asl

Ruth S. Slack
Acknowledgments
R.S.S. is supported by grants from the Canadian Institute of Health Research (CIHR) and the Heart and Stroke Foundation of Canada (HSFC). A.J.-A. is supported by a CIHR doctorate research award.
References
- Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M, Blackstone C (2005) Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol 15: 2112–2118 [DOI] [PubMed] [Google Scholar]
- Barsoum MJ et al. (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160: 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC (2006) Mitochondria: dynamic organelles in disease, aging and development. Cell 125: 1241–1252 [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C (2007) Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587 [DOI] [PubMed] [Google Scholar]
- Cheung EC, McBride HM, Slack RS (2007) Mitochondrial dynamics in the regulation of neuronal cell death. Apoptosis 12: 979–992 [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525 [DOI] [PubMed] [Google Scholar]
- Germain M, Mathai JP, McBride HM, Shore GC (2005) Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J 24: 1546–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J (2005) Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol 170: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R, Grote P, Westermann B, Conradt B (2005) DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433: 754–760 [DOI] [PubMed] [Google Scholar]
- Jahani-Asl A, Basak A, Tsang BK (2007a) Caspase-3-mediated cleavage of Akt: involvement of non-consensus sites and influence of phosphorylation. FEBS Lett 581: 2883–2888 [DOI] [PubMed] [Google Scholar]
- Jahani-Asl A, Cheung EC, Neuspiel M, Maclaurin JG, Fortin A, Park DS, McBride HM, Slack RS (2007b) Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem 282: 23788–23798 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S (2006) MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep 7: 1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39: 503–536 [DOI] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC (2006) Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol 26: 7397–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R (2004) Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 16: 59–68 [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529 [DOI] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177: 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MP (1999) The machinery of mitochondrial inheritance and behavior. Science 283: 1493–1497 [DOI] [PubMed] [Google Scholar]