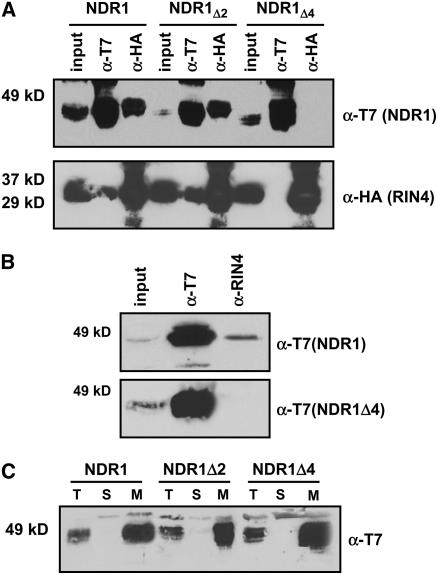
Figure 5.
The NDR1–RIN4 Interaction Occurs within the First Four Amino Acids of NDR1.
Stepwise deletions of two and four amino acids from the N terminus of NDR1 results in a differential pattern of association with RIN4.
(A) Coimmunoprecipitation and protein gel blot analysis of T7:NDR1 and HA:RIN4 from total protein extracts isolated from transiently expressed N. benthamiana. Loss of the NDR1–RIN4 interaction occurs following deletion of four amino acids from the N terminus of NDR1.
(B) Homologous expression of the NDR1Δ4 deletion mutant in Arabidopsis. Native promoter expression of T7:NDR1 and T7:NDR1Δ4 cDNAs in the Arabidopsis ndr1-1 mutant background confirms results of transient heterologous expression. Native RIN4 antibodies coimmunoprecipitate T7:NDR1 yet fail to coimmunoprecipitate T7:NDR1Δ4.
(C) Ultracentrifugation and localization of NDR1 and NDR1 deletion constructs confirm membrane localization. Total protein extracts from Arabidopsis plants (ndr1-1/HA:NDR1) expressing wild-type and deletion constructs were subjected to ultracentrifugation and separation by SDS-PAGE. Protein gel blot analysis (anti-T7) confirms plasma membrane localization for wild-type, Δ2, and Δ4 T7:NDR1 constructs.