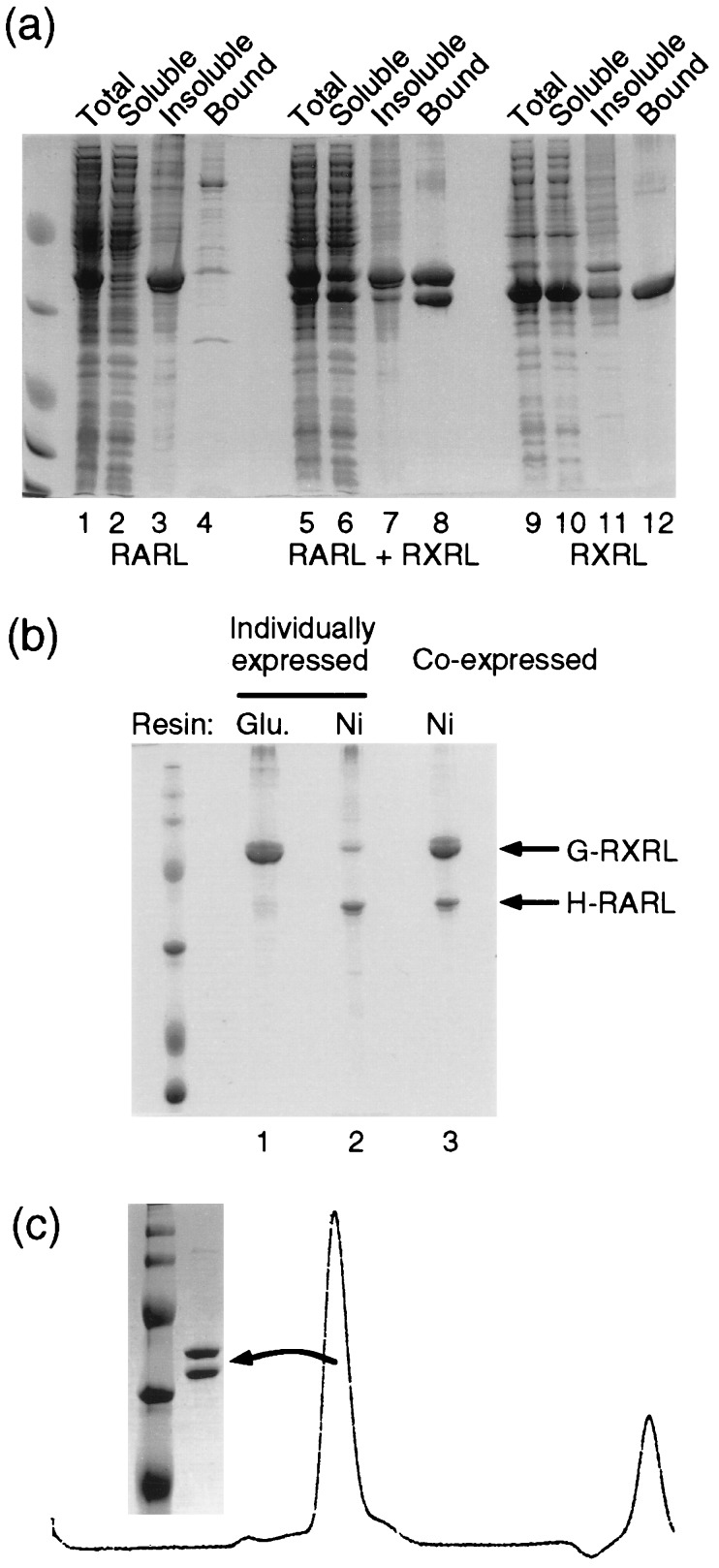
Figure 2.
Coexpression increases the solubility of RAR LBD and promotes RXR and RAR heterodimer formation. (a) Solubility analysis of individually expressed RAR and RXR LBDs and coexpressed RAR and RXR LBDs. All the RAR and RXR LBDs are tagged with six histidine residues at their N termini. The total cell lysates (Total), the soluble (Soluble) and insoluble (Insoluble) fractions, and the purified protein bound on the Ni2+ NTA beads (Bound) are indicated above each lane. (b) Heterodimer formation of individually or coexpressed RAR and RXR LBDs. Lane 1, the individually expressed H6 RAR LBD purified by interacting with GST RXR LBD on glutathione agarose beads; lane 2, the individually expressed GST RXR LBD was purified by interacting with the H6 RAR LBD on Ni2+ NTA agarose beads; lane 3, the coexpressed GST RXR LBD and H6 RAR LBD were purified by Ni2+ NTA agarose beads. (c) The coexpressed RXR and RAR LBDs are heterodimers. The coexpressed H6 RAR and RXR LBDs were passed through a gel filtration column. A single peak was observed at around the 56-kDa position. The proteins in the peak fraction were run on SDS/PAGE.