Abstract
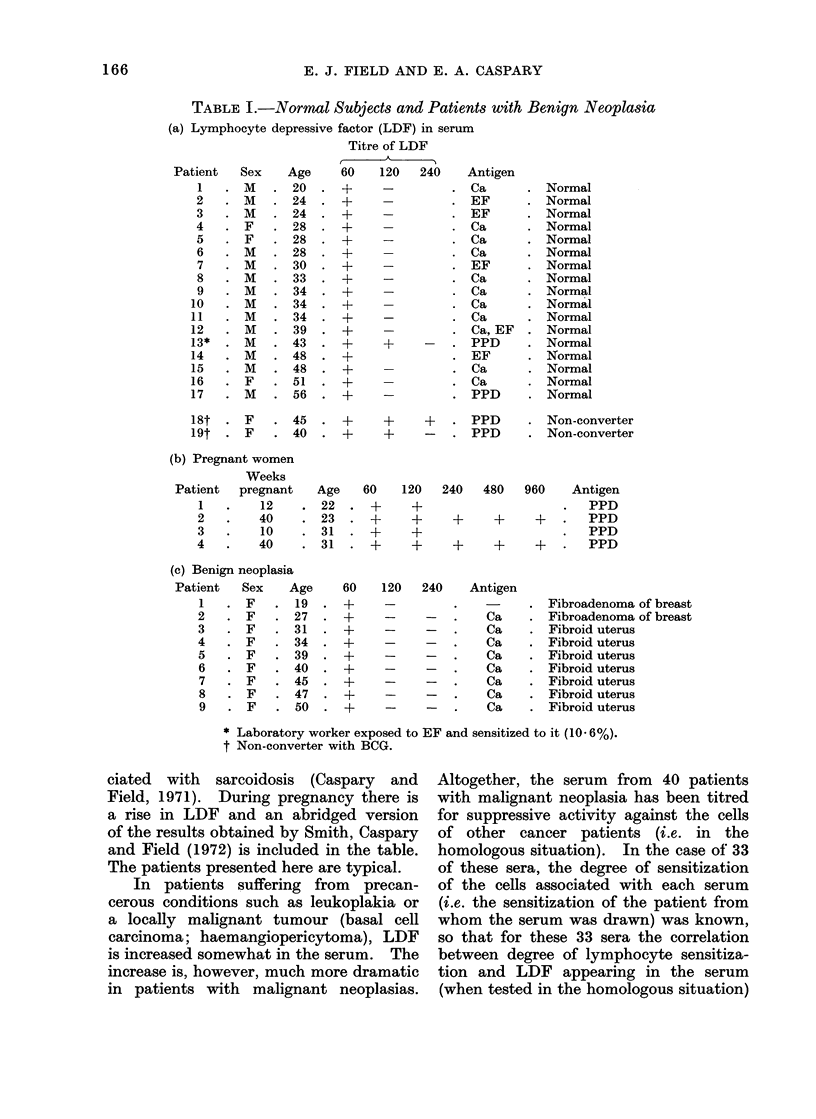
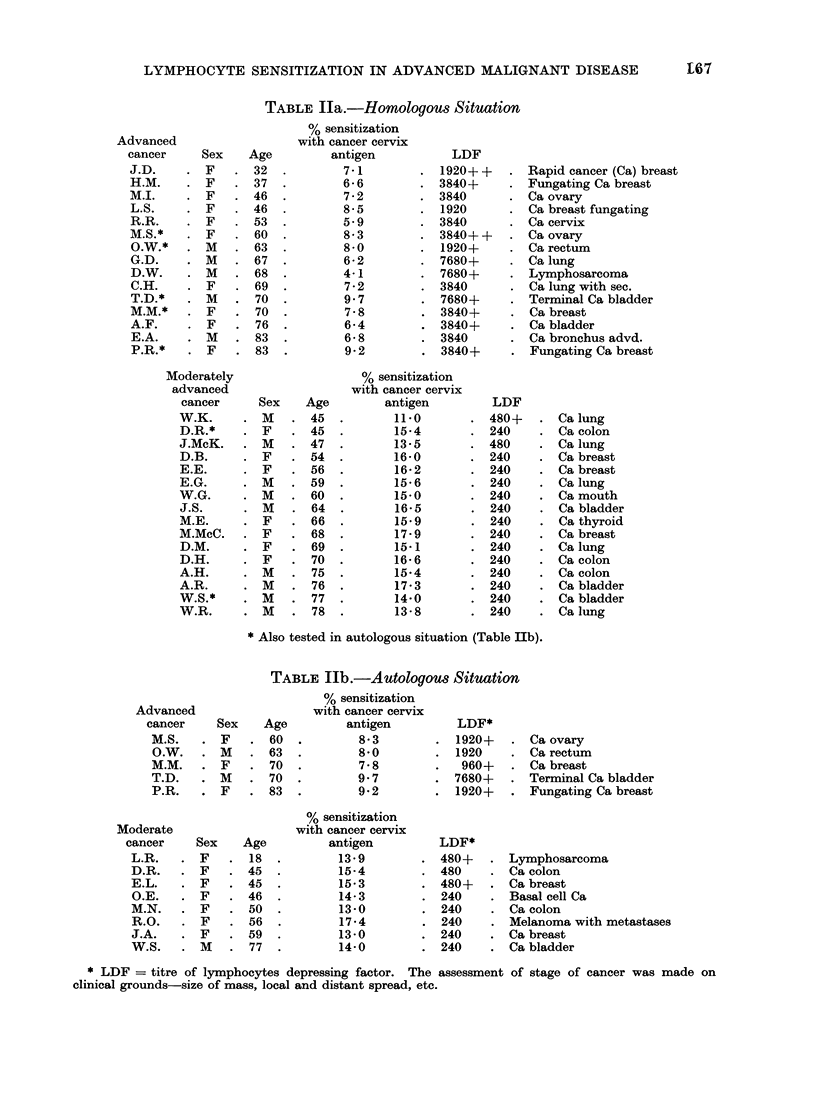
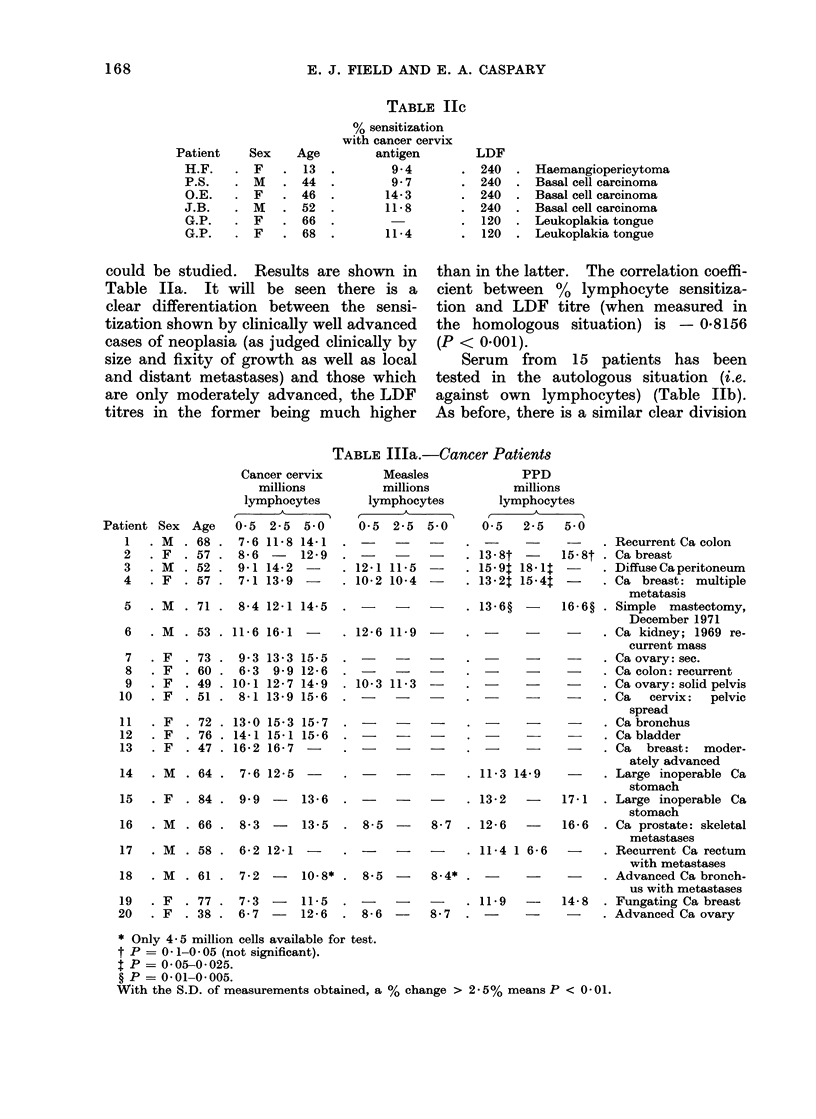
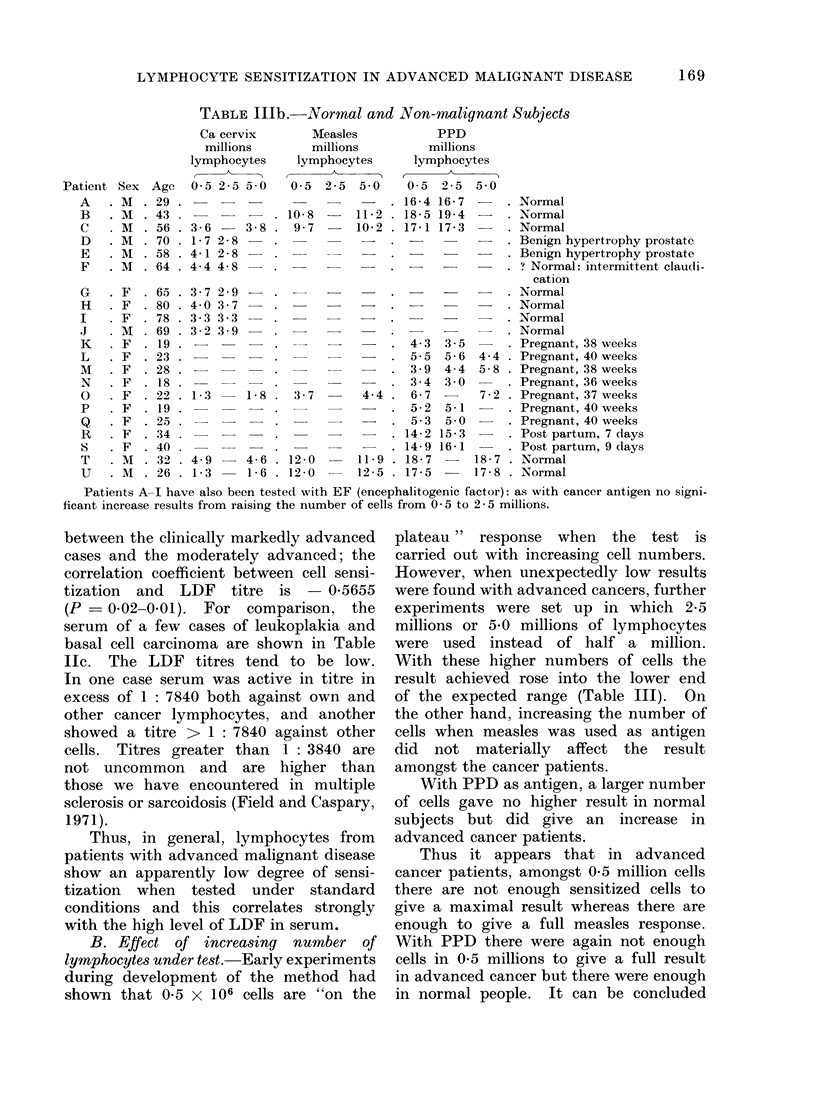
Patients with advanced malignant disease show an apparent lesser degree of lymphocyte sensitization to cancer antigen when tested under standard conditions than do early cases. In the serum of cancer patients there is a lymphocyte response depressing factor whose titre rises as the neoplasm becomes more extensive. The low lymphocyte response shown by advanced cancers is not, however, directly referable to this rise in depressive factor, but to removal by the tumour mass of specifically sensitized lymphocytes so that amongst the standard number of cells under routine test an adequate number does not remain to give a full response. Increasing the number of cells under test restores the result to the level found in moderately sized cancers. The “absorptive capacity” of large tumours for circulating sensitized lymphocytes is greater than can be provided by natural immunization produced by the tumour. Active immunization with a tumour antigen can be expected therefore to increase lymphocyte-associated defence against cancer.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benezra D., Hochman A. In vitro activation of lymphocytes from patients with malignant diseases. I. Kinetics and differences in magnitude of response. Isr J Med Sci. 1971 Apr;7(4):553–560. [PubMed] [Google Scholar]
- CASPARY E. A., FIELD E. J. AN ENCEPHALITOGENIC PROTEIN OF HUMAN ORIGIN: SOME CHEMICAL AND BIOLOGICAL PROPERTIES. Ann N Y Acad Sci. 1965 Mar 31;122:182–198. doi: 10.1111/j.1749-6632.1965.tb20202.x. [DOI] [PubMed] [Google Scholar]
- Caspary E. A., Field E. J. Specific lymphocyte sensitization in cancer: is there a common antigen in human malignant neoplasia? Br Med J. 1971 Jun 12;2(5762):613–617. doi: 10.1136/bmj.2.5762.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperband S. R., Bondevik H., Schmid K., Mannick J. A. Transformation of human lymphocytes: inhibition by homologous alpha globulin. Science. 1968 Mar 15;159(3820):1243–1244. doi: 10.1126/science.159.3820.1243. [DOI] [PubMed] [Google Scholar]
- FIELD E. J., CASPARY E. A., BALL E. J. Some biological properties of a highly active encephalitogenic factor isolated from human brain. Lancet. 1963 Jul 6;2(7297):11–13. doi: 10.1016/s0140-6736(63)92533-9. [DOI] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A., Carnegie P. R. Lymphocyte sensitization to basic protein of brain in malignant neoplasia: experiments with serotonin and related compounds. Nature. 1971 Sep 24;233(5317):284–286. doi: 10.1038/233284a0. [DOI] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A. Demonstration of sensitized lymphocytes in blood. J Clin Pathol. 1971 Mar;24(2):179–181. doi: 10.1136/jcp.24.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field E. J., Caspary E. A. Lymphocyte sensitisation: an in-vitro test for cancer? Lancet. 1970 Dec 26;2(7687):1337–1341. doi: 10.1016/s0140-6736(70)92361-5. [DOI] [PubMed] [Google Scholar]
- Hughes D., Caspary E. A., Field E. J. Lymphocyte trnsformation induced by encephalitogenic factor in multiple sclerosis and other neurological diseases. Lancet. 1968 Dec 7;2(7580):1205–1207. doi: 10.1016/s0140-6736(68)91690-5. [DOI] [PubMed] [Google Scholar]
- Hughes D., Caspary E. A. Lymphocyte transformation in vitro measured by tritiated thymidine uptake. I. Lymphocyte culture techniques. Int Arch Allergy Appl Immunol. 1970;37(5):506–531. doi: 10.1159/000230240. [DOI] [PubMed] [Google Scholar]
- Hughes D., Paty D. W. Macrophage migration inhibition test applied to delayed hypersensitivity in man. I. A mixed lymphocyte reaction between human and guinea-pig lymphocytes demonstrated using a sensitive micro-capillary method. Z Immunitatsforsch Exp Klin Immunol. 1972;142(5):478–487. [PubMed] [Google Scholar]
- KAMRIN B. B. Successful skin bomografts in mature non-littermate rats treated with fractions containing alpha-globulins. Proc Soc Exp Biol Med. 1959 Jan;100(1):58–61. doi: 10.3181/00379727-100-24522. [DOI] [PubMed] [Google Scholar]
- Knowles M., Hughes D., Caspary E. A., Field E. J. Lymphocyte transformation in multiple sclerosis. Inhibition of unstimulated thymidine uptake by a serum factor. Lancet. 1968 Dec 7;2(7580):1207–1209. doi: 10.1016/s0140-6736(68)91691-7. [DOI] [PubMed] [Google Scholar]
- Lehner T. Immunopathology of oral leukoplakia. Br J Cancer. 1970 Sep;24(3):442–446. doi: 10.1038/bjc.1970.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- MOWBRAY J. F. Ability of large doses of an alpha-2 plasma protein fraction to inhibit antibody production. Immunology. 1963 May;6:217–225. [PMC free article] [PubMed] [Google Scholar]
- MOWBRAY J. F. Effect of large doses of an alpha2-glycoprotein fraction on the survival of rat skin homografts. Transplantation. 1963 Jan;1:15–20. doi: 10.1097/00007890-196301010-00003. [DOI] [PubMed] [Google Scholar]
- Mathé G., Amiel J. L., Schwarzenberg L., Schneider M., Cattan A., Schlumberger J. R., Hayat M., De Vassal F. Active immunotherapy for acute lymphoblastic leukaemia. Lancet. 1969 Apr 5;1(7597):697–699. doi: 10.1016/s0140-6736(69)92648-8. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., Oppenheim J. J. Impaired lymphocyte transformation in ataxia-telangiectasia in part due to a plasma inhibitory factor. J Immunol. 1969 Dec;103(6):1212–1222. [PubMed] [Google Scholar]
- Mowbray J. F., Hargrave D. C. Further studies on the preparation of the immunosuppressive alpha-2 protein fraction from the serum and its assay in mice. Immunology. 1966 Nov;11(5):413–419. [PMC free article] [PubMed] [Google Scholar]
- Paronetto F., Popper H. Lymphocyte stimulation induced by halothane in patients with hepatitis following exposure to halothane. N Engl J Med. 1970 Aug 6;283(6):277–280. doi: 10.1056/NEJM197008062830602. [DOI] [PubMed] [Google Scholar]
- Riggio R. R., Schwartz G. H., Bull F. G., Stenzel K. H., Rubin A. L. Alpha-2-globulins in renal graft rejection. Effects of in vitro lymphocyte function. Transplantation. 1969 Nov;8(5):689–694. doi: 10.1097/00007890-196911000-00013. [DOI] [PubMed] [Google Scholar]
- Robinson E., Hurvitz D. In vitro studies of lymphocytes from cancer patients. Isr J Med Sci. 1966 Jan-Feb;2(1):80–81. [PubMed] [Google Scholar]
- Trubowitz S., Masek B., Del Rosario A. Lymphocyte response to phytohemagglutinin in Hodgkin's disease, lymphatic leukemia and lymphosarcoma. Cancer. 1966 Dec;19(12):2019–2023. doi: 10.1002/1097-0142(196612)19:12<2019::aid-cncr2820191228>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]


