Abstract
The in vitro interaction between activated, non-immune macrophages (AM) and a variety of syngeneic, allogeneic or xenogeneic “normal” and “malignant” target cell lines was followed by different parameters such as target cell proliferation, viability or morphology.
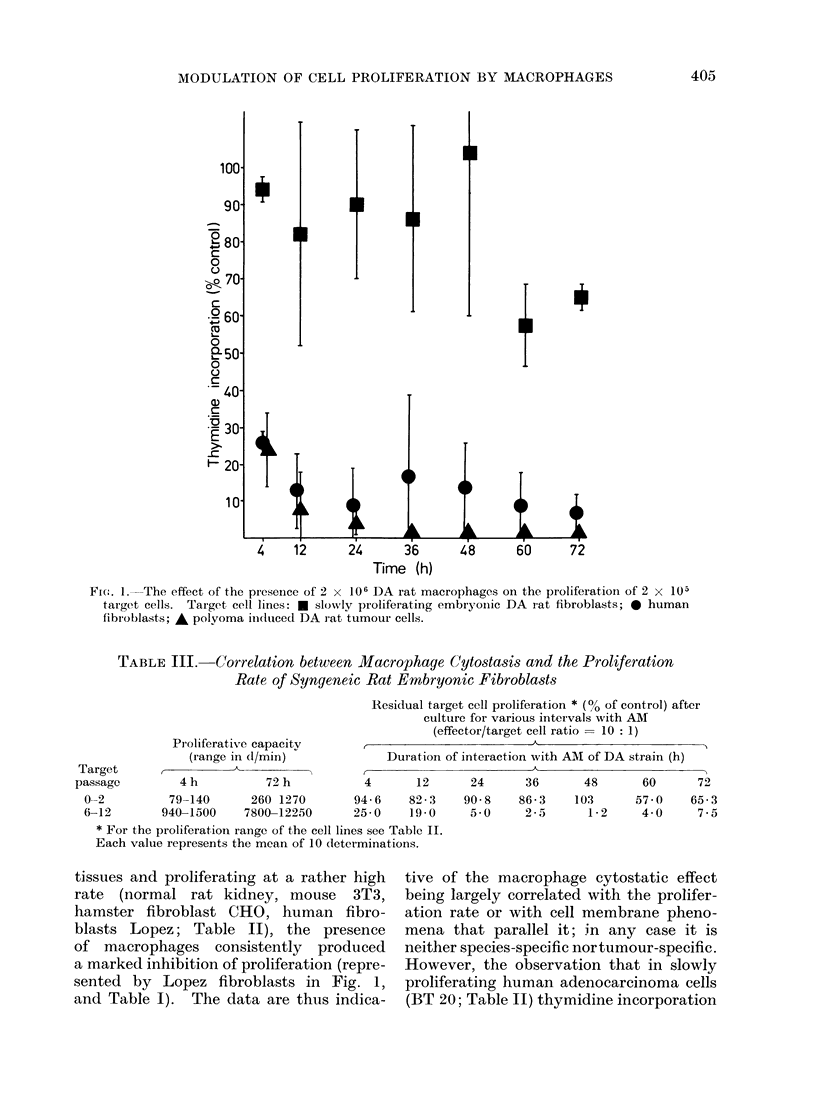
Proliferation of all rapidly replicating cell lines examined, irrespective of whether they were of syngeneic, allogeneic or xenogeneic origin, or showed normal or neoplastic growth characteristics, was similarly blocked by the presence of AM in an effector/target cell ratio of 10:1. It was only in very slowly proliferating cells that this inhibitory effect was not detectable. A marked diminution in target cell proliferation was also achieved with target cells growing in suspension, where maintenance of close contact between effectors and targets is unlikely, indicating that this macrophage effect may be mediated by a soluble product of AM.
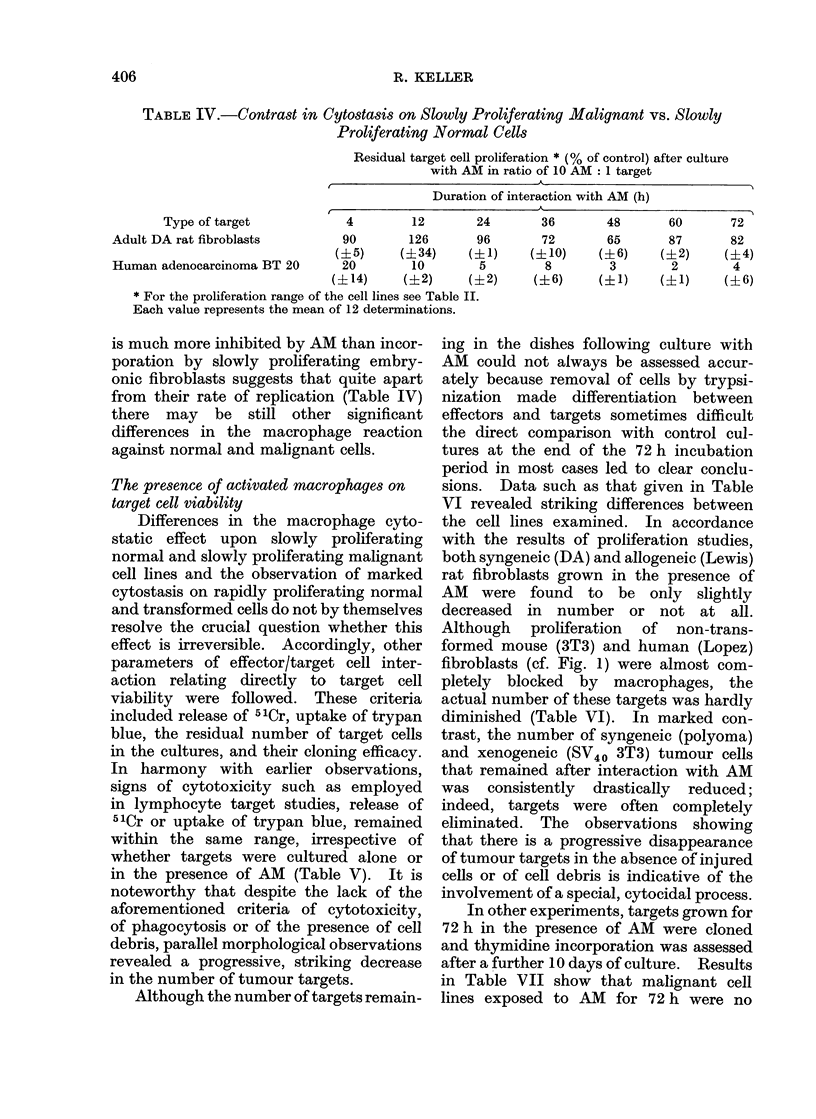
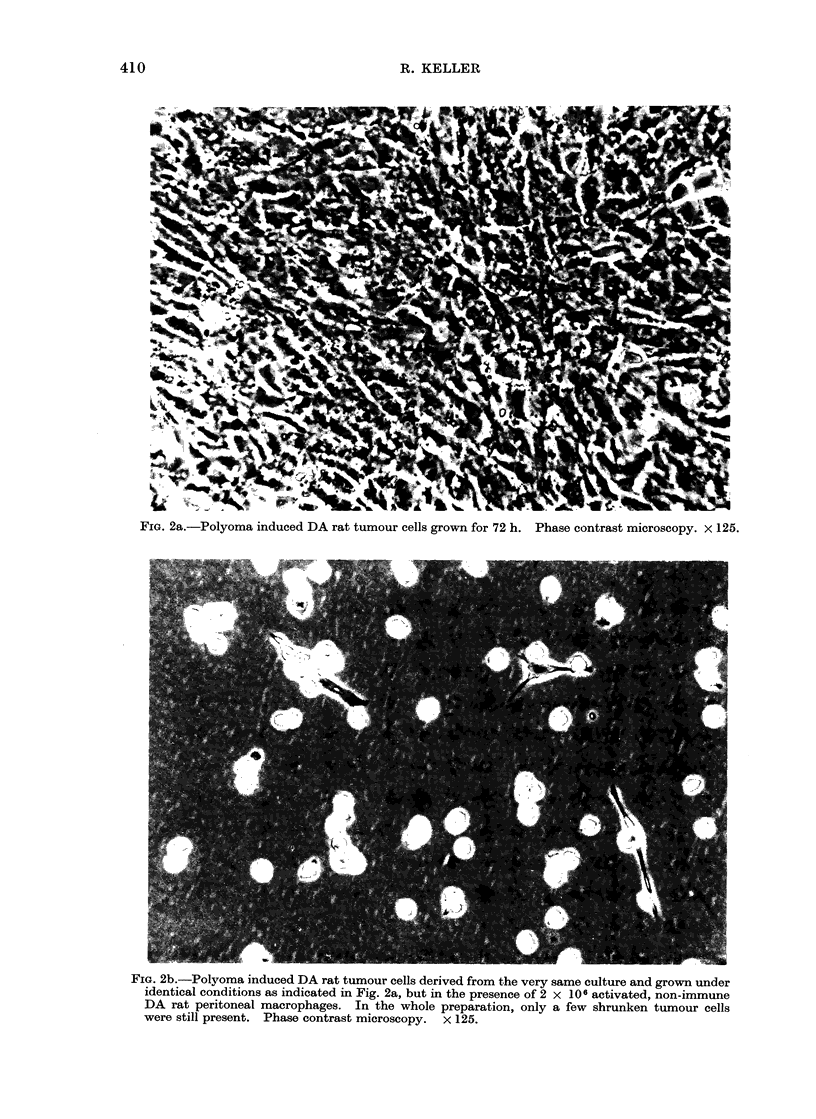
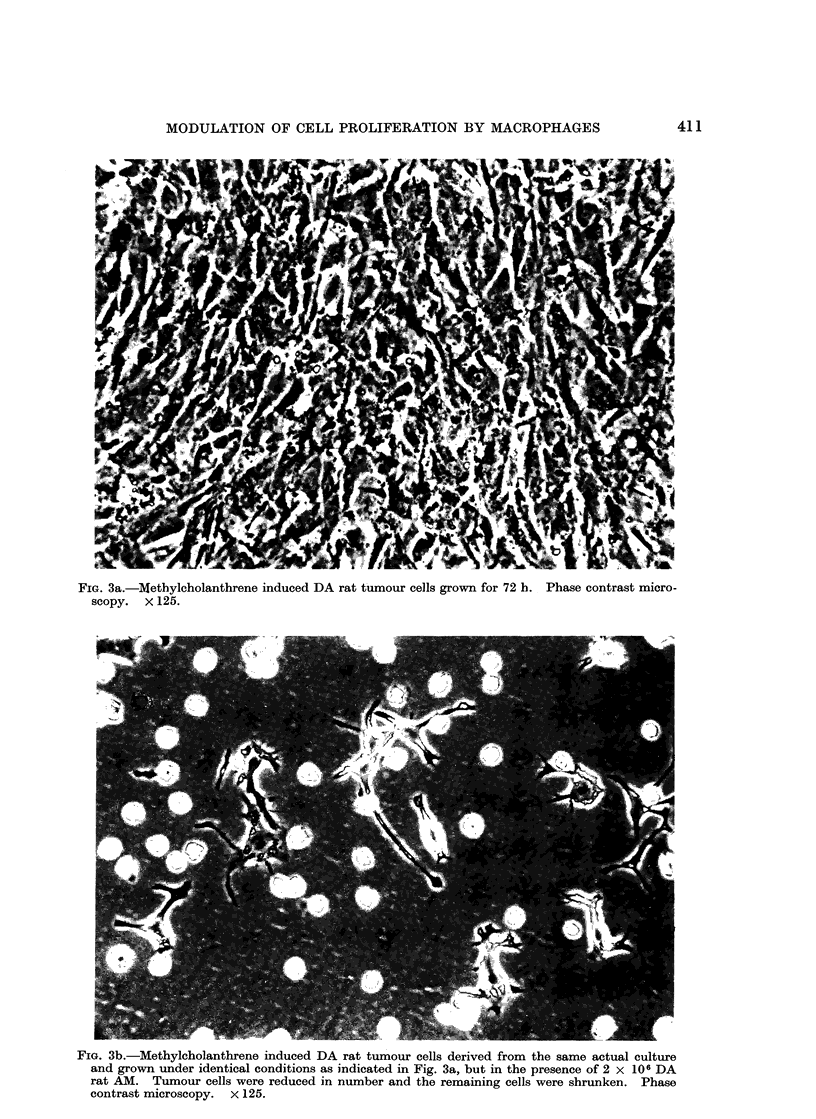
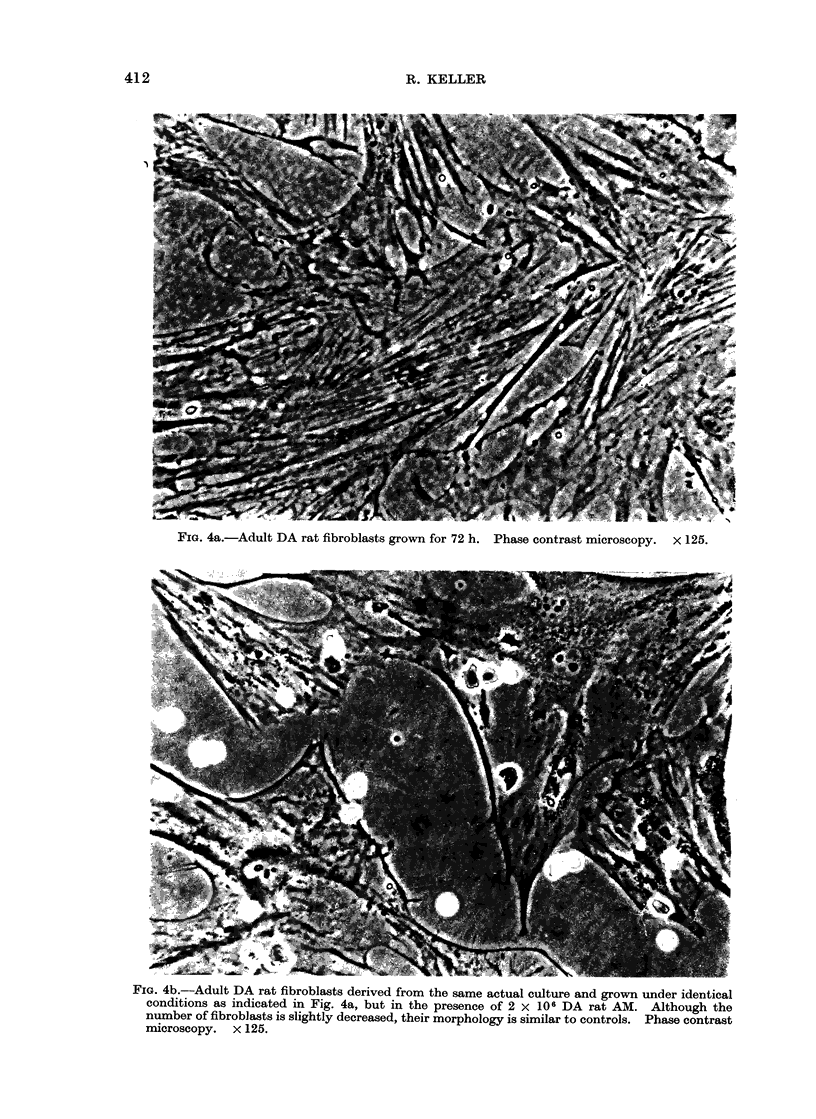
The finding of clear differences in the proliferation inhibition of slowly proliferating normal and neoplastic targets suggested that proliferation per se may not fully mirror the consequences of the macrophage/target cell interaction. This was affirmed when viability and morphology were used as parameters: viability was virtually unaffected in normal targets whereas neoplastic cells were killed.
Accordingly, it is suggested that activated non-immune macrophages can affect targets in strikingly different ways. Inhibition of proliferation could be an important homoeostatic regulatory function of the macrophage which would affect every replicating cell. Cytocidal killing of targets, on the other hand, is achieved only on neoplastic cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Diener E., Shortman K., Russell P. Induction of immunity and tolerance in vitro in the absence of phagocytic cells. Nature. 1970 Feb 21;225(5234):731–732. doi: 10.1038/225731a0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- GRANGER G. A., WEISER R. S. HOMOGRAFT TARGET CELLS: SPECIFIC DESTRUCTION IN VITRO BY CONTACT INTERACTION WITH IMMUNE MACROPHAGES. Science. 1964 Sep 25;145(3639):1427–1429. doi: 10.1126/science.145.3639.1427. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bourali C. Antitumor effects of interferon preparations in mice. J Natl Cancer Inst. 1970 Aug;45(2):365–376. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Holtermann O. A., Klein E., Casale G. P. Selective cytotoxicity of peritoneal leucocytes for neoplastic cells. Cell Immunol. 1973 Dec;9(3):339–352. doi: 10.1016/0008-8749(73)90049-x. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Cytostatic elimination of syngeneic rat tumor cells in vitro by nonspecifically activated macrophages. J Exp Med. 1973 Sep 1;138(3):625–644. doi: 10.1084/jem.138.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Evidence for compromise of tumour immunity in rats by a non-specific blocking serum factor that inactivates macrophages. Br J Exp Pathol. 1973 Jun;54(3):298–305. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Hess M. W. Tumour growth and non-specific immunity in rats: the mechanisms involved in inhibition of tumour growth. Br J Exp Pathol. 1972 Oct;53(5):570–577. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Jones V. E. Role of activated macrophages and antibody in inhibition and enhancement of tumour growth in rats. Lancet. 1971 Oct 16;2(7729):847–849. doi: 10.1016/s0140-6736(71)90222-4. [DOI] [PubMed] [Google Scholar]
- Keller R. Mechanisms by which activated normal macrophages destroy syngeneic rat tumour cells in vitro. Cytokinetics, non-involvement of T lymphocytes, and effect of metabolic inhibitors. Immunology. 1974 Aug;27(2):285–298. [PMC free article] [PubMed] [Google Scholar]
- Palm J. Immunogenetic analysis of Ag-B histocompatibility antigens in rats. Transplantation. 1971 Feb;11(2):175–183. doi: 10.1097/00007890-197102000-00011. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Dutton R. W. Inhibition of spleen cell DNA synthesis by autologous macrophages. J Immunol. 1966 Nov;97(5):663–669. [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Waldman S. R., Gottlieb A. A. Macrophage regulation of DNA synthesis in lymphoid cells: effects of a soluble factor from macrophages. Cell Immunol. 1973 Oct;9(1):142–156. doi: 10.1016/0008-8749(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Yoshinaga M., Yoshinaga A., Waksman B. H. Regulation of lymphocyte responses in vitro. I. Regulatory effect of macrophages and thymus-dependent (T) cells on the response of thymus-independent (B) lymphocytes to endotoxin. J Exp Med. 1972 Oct 1;136(4):956–961. doi: 10.1084/jem.136.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]