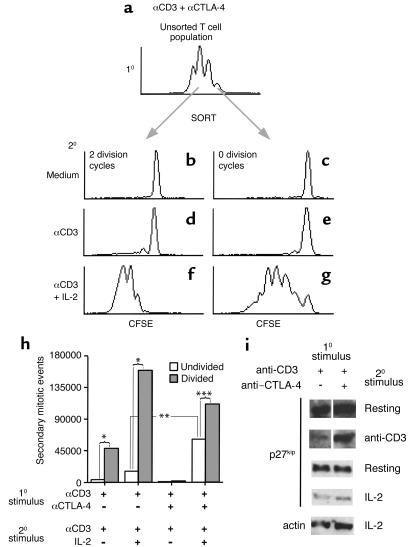
Figure 4.
Regulation of secondary T cell proliferation by CTLA-4–mediated signal transduction and cell division during the primary response. (a–h) CFSE-labeled splenocytes were stimulated with anti-CD3 in the presence of hamster IgG (first and second sets of columns in h), or either anti–CTLA-4 antibody (10 μg/ml; n = 2) or anti–CTLA-4 Fab (20 μg/ml; n = 2; a–g). Experiments using whole and Fab fragments of anti–CTLA-4 antibody gave similar results and are shown combined as n = 4 experiments (h, third and fourth sets of columns). Cultures were rested for 48 hours, and Thy1.2+ cells that had divided twice (b, d, and f; shaded bars in h) or had remained undivided (c, e, and g; open bars in h) following primary stimulation were purified by FACS. The sorted T cells were cultured with irradiated APCs and restimulated with anti-CD3 in the presence or absence of exogenous IL-2, and proliferation was assessed 4 days later by flow cytometry. One representative experiment is depicted graphically in a–g. The mean secondary mitotic events of separate experiments (n = 4, as described above) are plotted in h. The data from the control cultures (first and second sets of columns in h) are the same as depicted in Figure 1. Statistically significant differences were assessed by paired t test and are denoted by brackets: *P < 0.05; **P < 0.01; ***P < 0.001. (i) Lymph node and spleen cells were cultured with anti-CD3 in combination with anti-CD28 (first lane) or anti–CTLA-4 (second lane). The cultures were then rested for 24 hours (first panel), and a portion of the T cells were restimulated with anti-CD3–coated beads (second panel) for 48 hours. In a separate experiment, lymph node and spleen cells were stimulated as above, rested (third panel), and stimulated with IL-2 (50 U/ml; fourth panel) for 48 hours. Live cells were isolated over Ficoll, and lysates were subjected to immunoblot analysis using antibodies against p27kip1 or actin (fifth panel). The results shown are representative of two independent experiments.