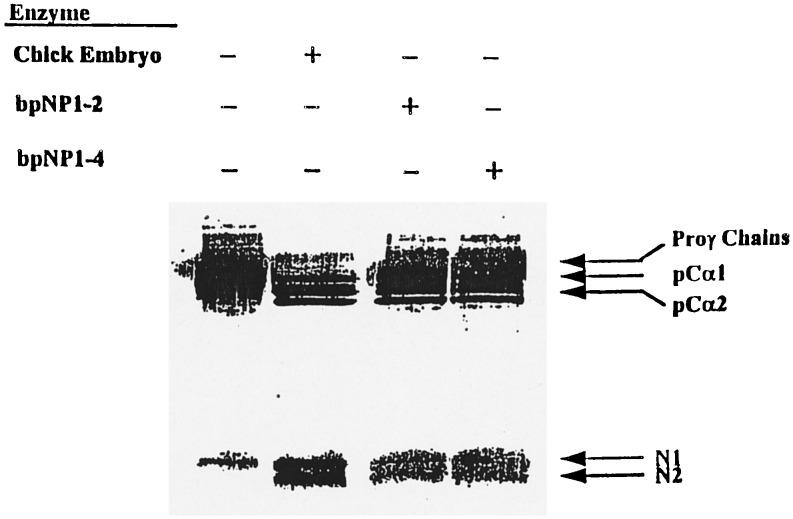
Figure 3.
Enzymatic activity of the recombinant bovine pNPI. Recombinant enzyme recovered from the conditioned medium of the two different HT-1080 clones expressing high level of pNPI mRNA were incubated with 14C-labeled procollagen substrate in the standard reaction buffer (16). After 4 h at 35°C, reaction products were separated by SDS/PAGE and visualized after autoradiography. bpNP1–2 and bpNP1–4, Enzyme from two different clones expressing a full-length cDNA for pNPI; Proγ chains, disulfide-linked proα chains of type I procollagen; pCα1 and pCα2, partially processed proα1(I) and proα2(I) chains of type I procollagen containing the C-propeptides but not the N-propeptides from proα1(I) and proα2(I) chains. N1 and N2, N-propeptides from proα1(I) and proα2(I) chains.