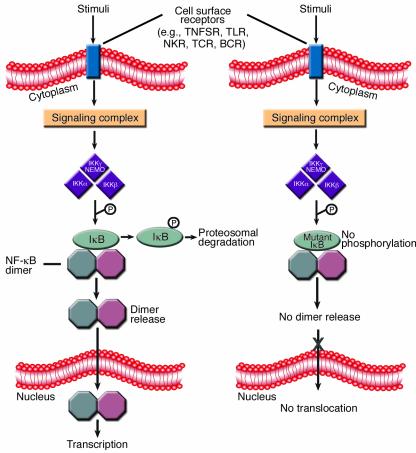
Figure 1.
Receptor-induced NF-κB nuclear translocation and inhibition by a dominant negative IκB. A variety of cell surface receptors are capable of inducing associated specific signaling complexes that can activate the IKK signalosome to phosphorylate IκB. This phosphorylation of IκB leads to its proteosomal degradation, thus liberating NF-κB dimers and allowing them to regulate gene transcription in the nucleus. The particular signaling complex activated and utilized by a given cell surface receptor varies and is specific to the receptor family. When a mutant IκB is present, the IKK signalosome is unable to phosphorylate the key serine residues, and NF-κB is retained in the cytoplasm bound to the mutant protein despite appropriate upstream activation. The octagons represent NF-κB family members, and individual dimers are frequently heterogeneous. NKR, NK cell activation receptor; TCR, T cell receptor; BCR, B cell receptor.