Abstract
Organ-specific autoimmune diseases have been postulated to be the result of T cell response against organ-specific self-peptides bound to MHC molecules. Contrary to this paradigm, we report here that transgenic mice lacking MHC class I expression and expressing an MHC class II I-Ab molecule that presents only a single peptide (Eα52-68) spontaneously develops peripheral nervous system–specific autoimmune disease with many of the histopathological features found in experimental allergic neuritis. Reciprocal bone marrow chimeras produced using susceptible and resistant lines revealed that bone marrow–derived cells determined disease susceptibility. While the expression of the I-Ab–Eα52-68 complex in the periphery was readily detectable in both lines, its expression on thymic dendritic cells responsible for tolerance induction was markedly lower in the susceptible line than in the resistant line. Consistent with this, CD4+ T cells that can be activated by the I-Ab–Eα52-68 complex were found in the susceptible line, but not in the resistant line. Such CD4+ T cells conferred the disease to the resistant line by adoptive transfer, and administration of Ab specific for the I-Ab–Eα52-68 complex inhibited disease manifestation in the susceptible line. These results indicate that disease development involves systemic T cell reactivity to I-Ab–Eα52-68 complex, probably caused by incomplete negative thymocyte selection.
Introduction
MHC class I and class II molecules are highly polymorphic glycoproteins that are expressed on the cell surface in association with self- or foreign antigenic peptides for recognition by CD8+ or CD4+ T cells, respectively (1). During development in the thymus, T cells are positively or negatively selected through the interaction of αβ T cell receptors (TCRs) with self-peptides bound to self-MHC molecules. Positive selection rescues immature T cells from programmed cell death and ensures that mature T cell repertoire is directed against foreign peptides bound to self-MHC molecules (2–5), whereas negative selection plays a central role in tolerance induction by eliminating potentially self-reactive T cells (6–9). Mature T cells, which survive these reciprocal selection processes and migrate to the periphery, respond to foreign peptides derived from bacteria, viruses, or parasites in association with self-MHC molecules expressed on antigen-presenting cells, and initiate immune response against invasion of such microorganisms. Thus, MHC molecules mediate two major immunological events, T cell repertoire selection in the thymus and immune response in the periphery, by presenting antigenic peptides to αβ TCRs.
A predisposition to organ-specific autoimmune diseases is associated with particular alleles of MHC loci (10). Since MHC molecules affect immune responsiveness to foreign antigens by presenting distinct sets of peptides to T cells through polymorphism at the peptide-binding groove (11–15), it has been postulated that efficient binding of organ-specific self-peptides by disease-associated MHC molecules leads to T cell–mediated organ-specific autoimmunity (16–18). However, the recent findings of increased overall self-reactivity of T cells in spontaneous mouse models of organ-specific autoimmune diseases have cast some doubt on this paradigm (19–21). The T cells involved in autoimmune processes are selected to mature on disease-associated MHC molecules via positive and negative selection in the thymus. Therefore, it is possible that MHC association with organ-specific autoimmune diseases reflects a T cell repertoire shaped by disease-associated MHC molecules bound to “unidentified” self-peptides in the thymus. However, this possibility has not been fully evaluated because of the difficulty in separately analyzing T cell repertoire selection and immune response in vivo.
Earlier, we reported on transgenic mouse lines that have been developed by introducing the gene encoding the I-Aβb chain covalently bound to Eα chain–derived peptide (Eα52-68) into mice lacking I-Aβb, invariant chain, and β2-microglobulin (TKO mice) (22–24). Thus far, no evidence has been provided that the I-Ab molecules expressed in such mice present self- or foreign antigenic peptides other than Eα52-68 to T cells (22, 25, 26), suggesting that I-Ab–Eα52-68 complex is the only MHC-peptide complex expressed in these mice. The expression of I-Ab–Eα52-68 complex was readily detected in the thymic cortex and medulla in one mouse line, B2L TKO, whereas the expression in another line, H3 TKO, was extremely low in the thymus (22, 24). However, CD4+ T cell differentiation was observed in both B2L TKO and H3 TKO mice (22, 24). Thus, these mice made it feasible to follow the immunological consequences of CD4+ T cells selected to mature on I-Ab–Eα52-68 complex expressed at different levels in the thymus, without these T cells being influenced by an immune response against other self- or foreign antigenic peptides.
Here we report the unexpected finding that H3 TKO mice spontaneously develop peripheral nervous system–specific autoimmune disease with many of the histopathological features found in experimental allergic neuritis, a model for Guillain-Barré syndrome (27, 28). By comparing H3 TKO mice with the resistant lines, B2L TKO mice and nontransgenic littermates (TKO mice), we demonstrate that disease development involves self-reactivity of CD4+ T cells to I-Ab–Eα52-68 complex, probably caused by its low expression on the thymic dendritic cells that are responsible for tolerance induction.
Methods
Mice.
Three lines of transgenic mice that express the I-Aβb chain covalently bound to Eα52-68 but lack endogenous I-Aβb, invariant chain, and/or β2-microglobulin have been described elsewhere (22–24). All mice were maintained under specific pathogen–free conditions, and mice hemizygous for the transgene were used in the present study.
Abs.
The following mAbs were purchased from PharMingen (San Diego, California, USA): FITC-conjugated anti-CD8 (53-6.7), FITC–anti-CD45R (RA3-6B2), FITC–anti-CD62L (MEL-14), phycoerythrin-conjugated (PE-conjugated) anti-CD4 (RM4-5), PE-conjugated anti–αβ TCR (H57-597), PE–anti-NK1.1 (PK136), PE–anti-CD90.2 (53-2.1), biotinylated anti-CD11b (M1/70), biotinylated anti–γδ TCR (GL3), biotinylated anti-CD44 (IM7), biotinylated anti-CD25 (7D4), and PE–anti-CD11c (HL3). YAe (an antibody against the I-Ab–Eα52-68 complex) (29) and Y3P (anti–I-Ab) (30) were isolated from supernatants of the hybridoma cell cultures using a protein G column, and the biotinylated Abs were used in the staining experiments. The mAb 33D1, specific for splenic dendritic cells, was purchased from Leinco Technologies Inc. (St. Louis, Missouri, USA).
Toluidine blue staining and electron microscopy.
Sciatic nerves were immersed in 2.5% glutaraldehyde in phosphate buffer for 14–16 hours at 4°C, and postfixed in 1% osmium tetroxide supplemented with 1.5% potassium ferrocyanide in phosphate buffer for 2 hours at room temperature. Samples were dehydrated in a graded ethanol series. Semithin sections were stained with toluidine blue and examined by light microscopy. Ultrathin sections were stained with lead citrate and uranyl acetate, and examined using a JEOL 1200 EX electron microscope (Japan Electron Optics Laboratory, Tokyo, Japan).
Immunohistochemistry.
Fresh-frozen tissue sections (6 μm thick) were prepared using a cryostat, and fixed in 100% acetone. Sections were stained with a panel of PE-conjugated mAbs in various combinations with biotinylated mAbs at 4°C for 14–16 hours. They were then washed with PBS, and incubated with Oregon Green 488–conjugated streptavidin (Molecular Probes, Eugene, Oregon, USA) for 3 hours at room temperature. Sections were then rinsed in PBS and viewed with a fluorescence microscope (Nikon Corp., Tokyo, Japan). Fluorescence and phase-contrast images were taken using the HCC-3800, a 3-CCD color camera from Flovel Co. Ltd. (Tokyo, Japan). Images were processed using Adobe Photoshop 5.0 (Adobe Systems Inc., San Jose, California, USA). To assess infiltration of CD11b+ macrophages into sciatic nerves, frozen sections were first incubated with biotinylated anti-CD11b mAb, then reacted with peroxidase-avidin. Sections were visualized in a fresh mixture of 0.02% 3,3′-diaminobenzidine in Tris-HCl buffer (0.005 M, pH 7.6).
Cell preparation and flow cytometry.
Splenic and thymic dendritic cells were prepared from spleens or thymi that were treated with collagenase D (Roche Molecular Biochemicals, Indianapolis, Indiana, USA), then centrifuged in dense BSA to provide a low-density population, and stained with YAe in combination with FITC-33D1 or FITC–anti-CD8 and PE–anti-CD11c mAbs, respectively. The expression of I-Ab–Eα52-68 complex on splenic B cells was assessed by staining spleen cells with FITC–anti-CD45R mAb and biotinylated YAe followed by streptavidin-PE. The cell-surface expression of CD44, CD62L, or CD25 on CD4+ T cells was analyzed by staining spleen cells with the relevant Abs. Analyses were done on a FACScan flow cytometer using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, California, USA).
CD4+ T cell proliferation and cytokine production.
Splenic CD4+ T cells were prepared from disease-affected H3 TKO mice using Dynabeads M-450 coated with anti-mouse CD4 mAb and DETACHaBEAD (both from Dynal Biotech, Oslo, Norway). These cells were cultured with irradiated B2H TKO spleen cells in the presence of 10% T-STIM culture supplement without ConA (Collaborative Biomedical Products, Bedford, Massachusetts, USA). After three or four rounds of stimulation, viable cells (1 × 105/well) recovered using Lympholyte-M (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) were cultured with irradiated spleen cells (1 × 106/well) from B2H TKO mice or TKO mice for 80 hours in the presence or absence of YAe at 20 μg/ml. During the final 16 hours of culture, 1 μCi of [3H]thymidine was added, and the incorporated radioactivity was measured. In some experiments, the production of IL-2, IL-4, or IFN-γ was measured using the Biotrak ELISA system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Bone marrow chimeras and adoptive transfer.
After eliminating mature T and B cells, bone marrow cells prepared from H3 TKO or B2L TKO mice were transferred into irradiated (850 cGy) B2L TKO, H3 TKO, or TKO mice (1 × 107 cells/recipient mouse). In some experiments, CD4+ peripheral lymph node cells (5–8 × 106) from the affected H3 TKO mice were stimulated with B2H TKO spleen cells in the presence of anti-CD3 mAb (1 μg/ml) and 10% T-STIM culture supplement without ConA, and transferred into the recipient mice at the time of reconstitution.
Ab administration.
One milligram of YAe or the isotype-matched Ab BB7.2, specific for HLA-A2, was intraperitoneally administered to H3 TKO or B2H TKO mice every 3–4 days, starting when mice were 7 weeks old. Sciatic nerves were prepared from H3 TKO mice at the age of 16 weeks and analyzed for infiltration of CD11b+ macrophages. For B2H TKO mice, the proportion of splenic B cells or dendritic cells expressing I-Ab–Eα52-68 complex was analyzed with flow cytometry.
Results
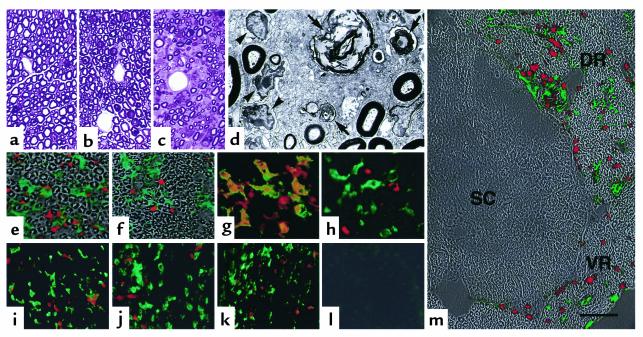
During the course of breeding, we found that H3 TKO mice exhibited dysfunction of their hind limbs. At 10 weeks old or later, they showed atony or clasping of hind limbs when lifted by the tail, and this dysfunction progressed gradually, with marked muscle atrophy. Thirty-three of fifty H3 TKO mice (66%) developed dysfunction of hind limbs and muscle atrophy by the age of 20 weeks. There was no significant difference in incidence between males and females (71.0% and 57.9%, respectively). In contrast, these clinical symptoms were not observed in B2L TKO mice or TKO mice. To visualize the pathological processes underlying the clinical manifestations of disease, we performed histological analysis. Each hind limb muscle from the affected H3 TKO mice was less than 60%, by weight, of that of nontransgenic littermates. The neuromuscular junctions did not show noticeable abnormalities in histology (data not shown). However, while compact myelinated axons were observed in the sciatic nerves of TKO and B2L TKO mice (Figure 1, a and b), the sciatic nerves from affected H3 TKO mice showed demyelination and axon degeneration with massive cell infiltration, especially around the blood vessels (Figure 1c). This became more evident with electron microscopic analysis, which showed that degenerated myelin was phagocytosed by macrophage-like cells rich in organelles, around which lymphoid cells gathered (Figure 1d). Immunohistochemical analysis revealed that the cells infiltrating the sciatic nerve of the affected H3 TKO mice expressed either CD4, CD11b, or NK1.1 (Figure 1, e and f). While CD4+ infiltrating cells also expressed αβ TCRs (Figure 1g), these cells were not stained with the mAb specific for NK1.1 (Figure 1h). Cells expressing CD8, γδ TCR, or CD45R were scarcely detected among the infiltrating cells (data not shown). Taken together, these results indicate that the sciatic nerves are infiltrated primarily by CD4+αβ TCR+ T cells and macrophages.
Figure 1.
Histopathology of the peripheral nervous system in affected H3 TKO mice. (a–c) The sciatic nerves from TKO (a), B2L TKO (b), and disease-affected H3 TKO mice (c) were stained with toluidine blue. (d) Electron micrograph of the same H3 TKO sciatic nerve shown in c, showing accumulation of lymphoid cells (arrowheads) around macrophages containing degenerated myelin sheaths within phagosomes (arrows). (e–h) Double immunofluorescence histochemistry of the sciatic nerve from an affected H3 TKO mouse shows infiltration of many CD11b+ cells (e and f, green) and CD4+ cells (e, red), and small numbers of NK1.1+ cells (f, red). Immunoreactivity for CD4 (g and h, green) is colocalized with that for αβ TCR (g, red), but not with that for NK1.1 (h, red). (i–m) The presence or absence of CD11b+ macrophages (green) and CD4+ T cells (red) in the facial (i), trigeminal (j), vagus (k), and optic (l) nerves, and in the spinal cord (SC), and dorsal (DR) and ventral (VR) roots of the spinal nerve (m) of affected H3 TKO mice. All peripheral nerve types sustain massive infiltration of CD4+ T cells and CD11b+ macrophages (i–k and m, DR and VR), whereas the central nervous tissues are intact (l and m, SC). Each fluorescence image is superimposed on the phase-contact image of the same field (e, f, and m). The scale bar represents 40 μm in a–c, 5 μm in d, 50 μm in e, f, and i–l, 25 μm in g and h, and 80 μm in m.
To assess organ specificity, we extended this analysis to other peripheral and central nerves as well as other organs of the affected H3 TKO mice. Although demyelination and axon degeneration were less severe in some peripheral nerves than in the sciatic nerve, infiltration of CD4+ T cells and macrophages was observed in the facial nerve, the trigeminal nerve, the vagus nerve, and the dorsal and ventral roots of the spinal nerve (Figure 1, i–k and m), indicating that the peripheral nervous system, irrespective of nerve location and function, is affected in H3 TKO mice. In contrast, the optic nerve, the spinal cord, and other organs including brain, liver, pancreas, kidney, and salivary glands, did not show any cell infiltration (Figure 1, l and m, and data not shown). These results indicate that the peripheral nervous system is strictly distinguished from other nerves and organs, and is specifically attacked in H3 TKO mice.
When the sciatic nerves of H3 TKO mice were analyzed within a week of clinical onset of symptoms, or at 20 weeks old in the case of the lack of clinical manifestations of disease, infiltration of CD11b+ macrophages was observed in almost all cases (Table 1). In contrast, the sciatic nerves of H3 TKO mice analyzed at 7 weeks old, and those of B2L TKO or TKO mice analyzed at 20 weeks old, did not show such cell infiltration (Table 1). Interestingly, however, while disease development was not observed with TKO mice that had been irradiated and reconstituted with H3 TKO bone marrow cells (H3→TKO chimera), B2L TKO mice similarly reconstituted with H3 TKO bone marrow cells (H3→B2L chimera) developed peripheral neuritis that was clinically and histopathologically equivalent to that seen in H3 TKO mice (Table 1). On the other hand, we found that reconstitution with B2L TKO bone marrow cells prevented H3 TKO mice from developing the disease (B2L→H3 chimera, Table 1). These results indicate that, in addition to thymic epithelial cells expressing I-Ab–Eα52-68 complex, bone marrow–derived cells from H3 TKO mice are required for disease development.
Table 1.
Histological analysis of the sciatic nerves from various mouse strains
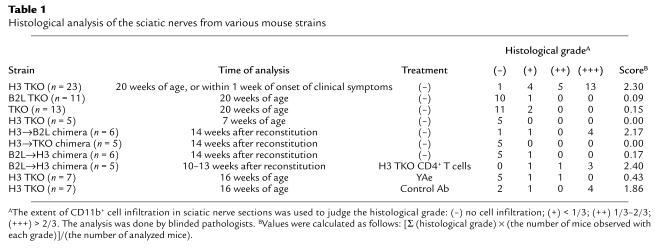
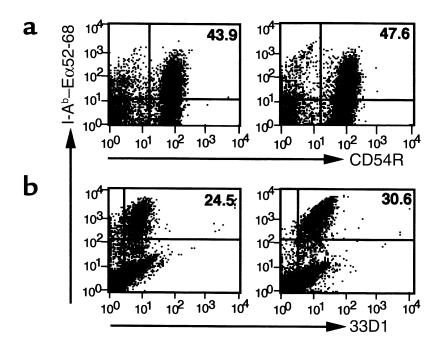
H3 TKO and B2L TKO mice differed markedly in expression levels of I-Ab–Eα52-68 complex in the thymic medulla (Figure 2a), where potentially self-reactive T cells are eliminated primarily through interaction with thymic dendritic cells (31–33). Having found that bone marrow–derived cells determine disease susceptibility, we compared the expression of I-Ab–Eα52-68 complex on thymic dendritic cells in H3 TKO and B2L TKO mice. Expression of I-Ab–Eα52-68 complex was markedly lower on thymic dendritic cells of H3 TKO mice than on those of B2L TKO mice (Figure 2b). However, expression of I-Ab–Eα52-68 complex was readily detected on splenic B cells and dendritic cells in both B2L TKO and H3 TKO mice (Figure 2, c and d).
Figure 2.
The expression of I-Ab–Eα52-68 complex on bone marrow–derived cells in the thymus and periphery. Thymus sections from TKO, H3 TKO, and B2L TKO mice were stained with YAe (a). The expression levels of I-Ab–Eα52-68 complex on CD11c+CD8+ thymic dendritic cells (b), CD45R+ splenic B cells (c), and 33D1+ splenic dendritic cells (d) were compared in H3 TKO and B2L TKO mice. The profiles of B2L TKO and H3 TKO mice (red) are shown with that of TKO mice (green).
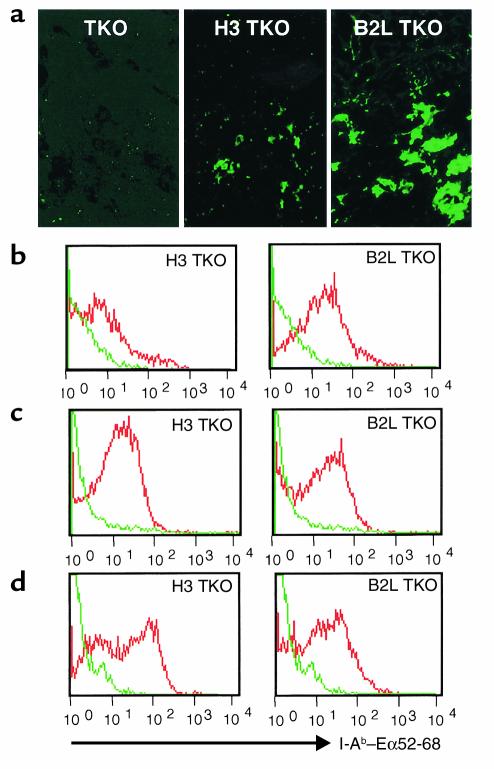
This raised the possibility that low expression of I-Ab–Eα52-68 complex on H3 TKO thymic dendritic cells results in incomplete negative selection and contributes to the disease development. To address this issue, we first compared immunological tolerance of CD4+ T cells to I-Ab–Eα52-68 complex in H3 TKO and B2L TKO mice. The proportion of splenic CD4+ T cells was 7.0% ± 1.2% in H3 TKO mice, whereas that in TKO and B2L TKO mice was 3.4% ± 0.5% and 11.5% ± 0.9%, respectively. While the expression of CD44 and CD62L on splenic CD4+ T cells from H3 TKO mice was intermediate between that in TKO and B2L TKO mice (Figure 3a), the proportion of CD4+CD25+ T cells to total splenic CD4+ T cells in H3 TKO mice was markedly higher than that in TKO or B2L TKO mice (Figure 3b). In primary culture, splenic CD4+ T cells from H3 TKO mice did not show any detectable proliferative response to I-Ab–Eα52-68 complex. This was the case even when the cells were stimulated with B2H TKO spleen cells expressing this complex as a single species at the highest level among the three lines (22) (data not shown). However, after three or four rounds of stimulation with B2H TKO spleen cells in the presence of cytokines, splenic CD4+ T cells from H3 TKO mice, but not those from B2L TKO mice, showed a definite proliferative response to B2H TKO spleen cells (Figure 3c). This response was not observed with TKO spleen cells and was blocked by the mAb YAe, which is specific for I-Ab–Eα52-68 complex (29). When CD4+ T cells from H3 TKO mice were stimulated with B2H TKO spleen cells, these cells produced IFN-γ and IL-4 (Figure 3d), but not IL-2 (data not shown). These results indicate that the peripheral T cell repertoire of H3 TKO mice, but not B2L TKO mice, includes self-reactive CD4+ T cells that can be activated by I-Ab–Eα52-68 complex.
Figure 3.
Phenotypic and functional analysis for self-reactivity of CD4+ T cells in H3 TKO mice. (a) Expression of CD62L and CD44 on splenic CD4+ T cells from TKO, H3 TKO, and B2L TKO mice. The proportions of CD62L+CD44–, CD62L+CD44+, and CD62L–CD44+ cells are indicated. (b) Expression of CD25 on splenic CD4+ T cells from TKO, H3 TKO, and B2L TKO mice (upper panels), and comparison of proportions of CD4+CD25+ splenic T cells in these three lines (lower panel). TKO, n = 4; H3 TKO, n = 7; B2L TKO, n = 4. (c and d) After three or four rounds of stimulation of splenic CD4+ T cells from H3 TKO (filled bars) or B2L TKO (open bars) mice with irradiated B2H TKO spleen cells in the presence of cytokines, viable cells (1 × 105/well) were cultured with irradiated spleen cells (1 × 106/well) from B2H TKO or TKO mice in the presence or absence of YAe (20 μg/ml). The results represent the mean of [3H]thymidine incorporation of duplicate cultures (c), and mean and SD of cytokine production of triplicate cultures (d). For the experiments shown in c and d, three independent short-term-cultured CD4+ T cell lines were prepared from disease-affected H3 TKO mice. B2H, B2H TKO.
We then explored the question of whether development of peripheral neuritis is mediated by CD4+ T cells in adoptive transfer experiments. For this purpose, we prepared CD4+ T cells from peripheral lymph nodes of H3 TKO mice that were afflicted with peripheral neuritis, and expanded the CD4+ T cells by stimulating them with B2H TKO spleen cells in the presence of anti-CD3 mAb and cytokines. While the sciatic nerves of B2L→H3 chimera mice did not show any cell infiltration (Table 1), inoculation with CD4+ T cells from H3 TKO mice affected with peripheral neuritis, together with B2L TKO bone marrow cells, resulted in infiltration of CD4+ T cells and CD11b+ macrophages in all cases (Table 1 and Figure 4). These results indicate that a CD4+ T cell–mediated immunological process is involved in development of peripheral neuritis.
Figure 4.
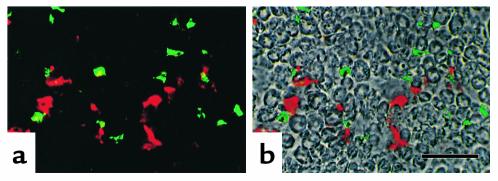
Adoptive transfer of CD4+ lymph node cells from disease-affected H3 TKO mice causes peripheral neuritis. Activated peripheral lymph node CD4+ T cells from affected H3 TKO mice were transferred into irradiated, 7-week-old H3 TKO mice, along with B2L TKO bone marrow cells. Sciatic nerve sections were prepared and stained for CD11b (green) and CD4 (red) at 10–13 weeks after the transfer. A double immunofluorescence image (a) is superimposed on the phase-contrast image of the same field (b). The scale bar represents 40 μm.
In H3 TKO mice, MHC class II I-Ab molecules, which serve as ligands for TCRs expressed on CD4+ T cells, form complexes with Eα52-68. To investigate whether peripheral neuritis involves self-reactivity to the I-Ab–Eα52-68 complex, we administered 1 mg of the mAb YAe to H3 TKO mice every 3–4 days, starting at the age of 7 weeks. While four of seven H3 TKO mice similarly treated with the isotype-matched control Ab developed peripheral neuritis by the age of 16 weeks, none of those treated with YAe exhibited clinical manifestations of disease. When the sciatic nerves were analyzed for CD11b+ cell infiltration at 16 weeks of age, YAe administration was found to markedly improve the histological score (Table 1). It is clear that the suppressive effect of YAe on disease development does not result from elimination of antigen-presenting cells, because the proportions of dendritic cells and B cells expressing I-Ab–Eα52-68 complex in the spleen were not changed much by YAe treatment (Figure 5).
Figure 5.
Comparison of B cells and dendritic cells expressing I-Ab–Eα52-68 complex before and after YAe treatment. Spleen cells (a) and dendritic cell–enriched fractions (b) were prepared from B2H TKO mice with (left) or without (right) YAe treatment, and stained with YAe and anti-CD45R mAb (for detection of B cells) or 33D1 (for detection of splenic dendritic cells). The proportions of splenic B cells and dendritic cells expressing I-Ab–Eα52-68 complex are indicated in upper right hand corners.
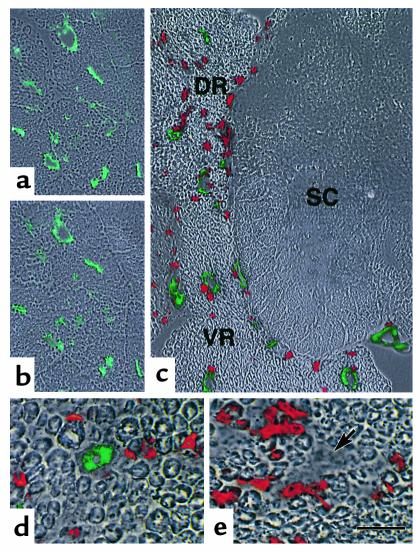
This finding led us to closely examine the expression of I-Ab–Eα52-68 complex using two mAbs: YAe, which is specific for I-Ab–Eα52-68 complex (29), and Y3P, which reacts with I-Ab molecules irrespective of the binding peptide (30). While no expression of I-Ab–Eα52-68 complex was detected in muscle, liver, pancreas, or kidney (data not shown), both YAe and Y3P strongly stained the vascular endothelium of the sciatic nerves of H3 TKO mice afflicted with peripheral neuritis (Figure 6, a and b). Since I-Ab molecules that were stained with Y3P, but not with YAe, were not found in the serial sections, it seems that I-Ab molecules on the vascular endothelium are occupied with Eα52-68 at this level of detection. This is similar to the situation with antigen-presenting cells (22, 25, 26). When the spinal nerve was analyzed, strong expression of I-Ab–Eα52-68 complex was observed on the vascular endothelium of the dorsal and ventral roots. The complex was not found on vascular endothelium of the spinal cord (Figure 6c). Similar results were obtained with B2L→H3 chimeras inoculated with H3 TKO CD4+ T cells (Figure 6d). On the other hand, the expression of I-Ab–Eα52-68 complex was not detected in the peripheral nervous tissues from 7-week-old H3 TKO mice or 20-week-old B2L TKO mice, in which no cell infiltration was detected (data not shown). However, we found that sciatic nerve sections from H3→B2L chimera did not express I-Ab–Eα52-68 complex on the vascular endothelium, despite infiltration of CD4+ T cells (Figure 6e).
Figure 6.
Expression of I-Ab–Eα52-68 complex on the vascular endothelium of peripheral nerves from disease-affected H3 TKO mice. (a and b) Serial sciatic nerve sections from affected H3 TKO mice with peripheral neuritis were stained with YAe (a, green) and Y3P (b, green). (c) Fresh-frozen sections from affected H3 TKO mice including the dorsal and ventral roots of the spinal nerve (DR and VR) and the spinal cord (SC) were stained with YAe (green) and anti-CD4 mAb (red). (d) Sciatic nerve sections from B2L→H3 chimeras inoculated with H3 TKO CD4+ T cells were stained with YAe (green) and anti-CD4 mAb (red). (e) Sciatic nerve sections from H3→B2L chimeras were stained with YAe (green) and anti-CD4 mAb (red). The I-Ab–Eα52-68 complex is not expressed in the vascular walls (arrow). Each fluorescence image was superimposed on the phase-contrast image of the same field (a–e). The scale bar represents 100 μm in a and b, 80 μm in c, and 25 μm in d and e.
Discussion
In the present study, we have shown that a transgenic mouse line, H3 TKO, which expresses I-Ab molecule covalently bound to Eα52-68 as its only MHC-peptide complex, spontaneously develops a demyelinating peripheral neuritis with infiltration of numerous CD4+αβ TCR+ T cells and macrophages. Such neuritis was not observed in TKO and B2L TKO mice. However, reconstitution with H3 TKO bone marrow cells conferred the disease to B2L TKO mice, but not to TKO mice, suggesting that CD4+ T cells selected to mature on I-Ab–Eα52-68 complex are required for disease development. This was shown more directly by another finding: while peripheral neuritis did not develop in B2L→H3 chimeras, inoculation of CD4+ T cells from affected H3 TKO lymph nodes induced a similar pathological change in this line. These results suggest that the peripheral neuritis observed in H3 TKO mice results from CD4+ T cell–mediated autoimmunity, rather than being a result of transgene position or an undetected infection.
The disease-susceptible line, H3 TKO, was originally developed in a double-knockout genetic background that lacks I-Aβb and invariant chains, but expresses endogenous MHC class I molecules H3 DKO(22); we had never seen development of peripheral neuritis in this background (data not shown). Lack of the disease development in H3 DKO mice might be the result of immune regulation in the periphery by CD8+ T cells or CD4+NK1.1+ natural killer T cells (NKT cells), the development of which requires classical or nonclassical MHC class I molecules (34, 35); or it could be due to elimination of the pathogenic CD4+ T cells by MHC class I molecules in the thymus. Alternatively, MHC class I expression might suppress self-reactivity of CD4+ T cells by simply increasing the number of T cells, because it has been reported that reducing the total size of the T cell pool causes native T cells to become overtly reactive to positively selecting peptide ligands (36, 37). Although the mechanism for disease inhibition by endogenous MHC class I expression awaits further study, this also argues against the involvement of a position effect of the transgene in disease development.
Immune responsiveness is determined by MHC molecules through T cell repertoire selection in the thymus and antigen presentation in the periphery. However, since MHC class I expression is lacking and MHC class II I-Ab molecules are occupied with Eα52-68 in both H3 TKO and B2L TKO mice, it is likely that the differential susceptibility of these lines to autoimmune disease is attributable to T cell repertoire selection directed by I-Ab–Eα52-68 complex that is expressed in the thymus at different levels. Reciprocal bone marrow chimeras of H3 TKO and B2L TKO mice revealed that bone marrow–derived cells from H3 TKO mice are critical for disease development. While the expression of I-Ab–Eα52-68 complex in the peripheral antigen presenting cells of H3 TKO mice was comparable to or somewhat higher than that expression in B2L TKO mice, its expression on the thymic dendritic cells responsible for tolerance induction was markedly lower in H3 TKO mice than in B2L TKO mice. Consistent with this, the mature T cell repertoire of H3 TKO mice, but not of B2L TKO mice, included CD4+ T cells that were reactive to I-Ab–Eα52-68 complex. We have also shown that administration of YAe inhibits manifestation of the autoimmune disease without eliminating antigen-presenting cells. Taken together, these results strongly suggest that incomplete negative selection directed by low-expressing of I-Ab–Eα52-68 complex in the thymus, and the resultant “leakiness” of self-reactivity to this selecting ligand, are involved in disease development. At this stage, however, it remains unclear how self-reactive CD4+ T cells are activated in the periphery, and how such activation leads to organ-specific autoimmune disease in H3 TKO mice. It may be that the activation of self-reactive CD4+ T cells is a result of the unbalanced expression of I-Ab–Eα52-68 complex in the thymus and periphery in this line; that is, extremely low expression on thymic dendritic cells and relatively high expression on peripheral antigen-presenting cells.
Ridgeway et al. reported that immunological tolerance of T cells to several self-peptides, irrespective of organ specificity, is insufficient in nonobese diabetic (NOD) mice, a model for type I diabetes, and suggested that this can be attributed to negative selection directed by I-Ag7 (20, 21). A more convincing case for involvement of incomplete negative selection in organ-specific autoimmune diseases was made by Kouskoff et al., who showed that the transgenic KRN mouse line, expressing αβ TCR with cross-reactivity to I-Ag7, developed joint-specific autoimmune disease when crossed with mice expressing I-Ag7 (19). Our findings using H3 TKO mice, together with results from studies of NOD and KRN mice, raise the possibility that many of the spontaneous organ-specific autoimmune diseases are provoked by systemic self-reactivity of T cells — reactivity that is generated through T cell repertoire selection that is directed by disease-associated MHC molecules in the thymus. The present study suggests that T cell repertoire selection directed by low expressioning MHC–self-peptide complex causes such systemic T cell self-reactivity. A similar situation might occur with T cells selected by unstable MHC–self-peptide complexes. Regarding these possibilities, it is interesting to note that I-Ag7, which determines susceptibility to the autoimmune diseases in NOD and KRN mice, has been reported as a poor or promiscuous peptide binder that may lack high-affinity binding peptides (38, 39).
The results from H3 TKO and KRN mice resemble each other in that these mice, which have limited diversity of TCR ligands or TCRs, spontaneously develop organ-specific autoimmune disease as a consequence of the systemic self-reactivity of CD4+ T cells. However, the processes leading to organ-specific autoimmunity are different in these two models. Disease onset in KRN is acute (19), whereas H3 TKO mice take a chronic course. Joint-specific autoimmune disease in KRN mice was shown to involve humoral response to a ubiquitously expressed self-antigen, glucose-6-phosphate isomerase (40, 41). However, serum from H3 TKO mice with peripheral neuritis could neither stain sciatic nerve sections nor transfer the disease (data not shown). On the other hand, we found that I-Ab–Eα52-68 complex was strongly expressed on the vascular endothelium of the peripheral nervous system of the affected mice. Since sciatic nerve sections from H3→B2L chimera showed cell infiltration without expression of I-Ab–Eα52-68 complex on the vascular endothelium, it seems likely that such expression is the result of inflammation in nearby parenchymal tissue, rather than being the cause of the disease. Nonetheless, it is conceivable that I-Ab–Eα52-68 complex expressed on the vascular endothelium could be involved in disease manifestation by facilitating the entry of self-reactive CD4+ T cells into the tissue and/or inducing cytokine secretion by them.
In conclusion, we have provided a novel mouse model for organ-specific autoimmune diseases in which peripheral nervous tissues are specifically attacked as a consequence of systemic self-reactivity of CD4+ T cells. Our findings suggest that the expression level of MHC–self-peptide complexes in the thymus, especially on thymic dendritic cells, contributes to susceptibility to organ-specific autoimmune disease by determining the size and affinity of the self-reactive T cell repertoire.
Acknowledgments
We thank D.B. Murphy and M. Kimoto for providing us with the B cell hybridomas YAe and Y3P, respectively. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Japan Science and Technology Corporation.
Footnotes
Takamasa Oono and Yoshinori Fukui contributed equally to this work.
References
- 1.Germain RN. MHC-dependent antigen processing and peptide presentation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Teh HS, Blüthman H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 3.Sha WC, et al. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 4.Teh H-S, et al. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:119–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 5.Berg LJ, et al. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 6.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald HR, et al. T-cell receptor Vβ use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988;332:40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 8.Kisielow P, Blüthman H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 9.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 10.Todd JA, et al. A molecular basis for MHC class II-associated autoimmunity. Science. 1988;240:1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- 11.Benacerraf B, McDevitt HO. Histocompatibility-linked immune response genes. Science. 1972;175:273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- 12.Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenetic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 13.Sette A, et al. Structural characteristics of an antigen required for its interaction with Ia and recognition by T cells. Nature. 1987;328:395–399. doi: 10.1038/328395a0. [DOI] [PubMed] [Google Scholar]
- 14.Guillet J-G, et al. Immunological self, nonself discrimination. Science. 1987;235:865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- 15.Rammensee H-G, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 16.Todd JA, Bell JI, McDevitt HO. HLA-DQβ gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 17.Sinha AA, Lopez MT, McDevitt HO. Autoimmune diseases: the failure of self-tolerance. Science. 1990;248:1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- 18.Theofilopoulos AN. The basis of autoimmunity: part I. Mechanisms of aberrant self-recognition. Immunol Today. 1995;16:90–98. doi: 10.1016/0167-5699(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 19.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 20.Ridgeway WM, Fassó M, Lanctot A, Garvey C, Fathman CG. Breaking self-tolerance in nonobese diabetic mice. J Exp Med. 1996;183:1657–1662. doi: 10.1084/jem.183.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridgeway WM, Ito H, Fassó M, Yu C, Fathman CG. Analysis of the role of the variation of major histocompatibility complex class II expression on nonobese diabetic (NOD) peripheral T cell response. J Exp Med. 1998;188:2267–2275. doi: 10.1084/jem.188.12.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui Y, et al. Positive and negative CD4+ thymocyte selection by a single MHC class II/peptide ligand affected by its expression level in the thymus. Immunity. 1997;6:401–410. doi: 10.1016/s1074-7613(00)80283-6. [DOI] [PubMed] [Google Scholar]
- 23.Fukui Y, et al. Highly restricted T cell repertoire shaped by a single major histocompatibility complex-peptide ligand in the presence of a single rearranged T cell receptor β chain. J Exp Med. 1998;188:897–907. doi: 10.1084/jem.188.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukui Y, et al. Diversity of T cell repertoire shaped by a single peptide ligand is critically affected by its amino acid residue at a T cell receptor contact. Proc Natl Acad Sci USA. 2000;97:13760–13765. doi: 10.1073/pnas.250470797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 26.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 27.Waksman BH, Adams RD. Allergic neuritis: an experimental disease of rabbits by the injection of peripheral nervous tissue and adjuvants. J Exp Med. 1955;102:213–236. doi: 10.1084/jem.102.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn AF. Experimental allergic neuritis (EAN) as a model for the immune-mediated demyelinating neuropathies. Rev Neurol. 1996;5:328–332. [PubMed] [Google Scholar]
- 29.Rudensky A, Rath S, Preston-Hurburt P, Murphy D, Janeway CA., Jr On the complexity of the self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 30.Janeway CA, Conrad P, Lerner E, Babich J, Wettstein P. Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells: possible role of T cell-bound Ia antigens as targets of immunoregulatory T cells. J Immunol. 1984;132:662–669. [PubMed] [Google Scholar]
- 31.Marrack P, et al. The effect of thymus environment on T cell development and tolerance. Cell. 1988;53:627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- 32.Sprent J, Lo D, Gao E-K, Ron Y. T cell selection in the thymus. Immunol Rev. 1988;101:173–189. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 33.Laufer TM, Dekoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 34.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 35.Bendelac A, et al. CD1 recognition by mouse NK1+ thymocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 36.Ernst B, Lee D-P, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 37.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco-Martin E, Shimizu J, Kanagawa O, Unanue ER. The class II MHC I-Ag7 molecules from non-obese diabetic mice are poor peptide binders. J Immunol. 1996;156:450–458. [PubMed] [Google Scholar]
- 39.Stratmann T, et al. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J Immunol. 2000;165:3214–3225. doi: 10.4049/jimmunol.165.6.3214. [DOI] [PubMed] [Google Scholar]
- 40.Korganow A-S, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]