Abstract
Cycasin (methylazoxymethanol-beta-D-glucoside) is carcinogenic in several animal species. It produces a variety of malignant tumours, mainly in the liver of mice, and in the liver, kidney and large intestine in rats. It does not appear to be mutagenic in the Ames test, even in the presence of liver microsome fraction, and it is among those carcinogens (less than 10%) ranked as "false negatives" in this test. The ability of cycasin to damage in vivo liver, kidney, lung and colonic DNA of Wistar rats and C57BL/L mice was investigated by means of alkaline elution technique. Oral single-dose administration of cycasin, in the range of 50-400 mg/kg body weight, produced in the rat a clearly evident dose-dependent DNA fragmentation in the liver, and less marked damage to DNA from kidney and colon mucosa. In mice, the same treatment produced dose-dependent DNA damage only in the liver. DNA repair up to 18 h appeared to be incomplete both in mice and rats. Methylazoxymethanol acetate is considered to be an active form of cycasin. While in vivo methylazoxymethanol acetate caused DNA damage, in vitro it appeared inactive and required metabolic activation, possibly consisting in its hydrolysis by esterase activity, to be able to cause DNA fragmentation.
Full text
PDF

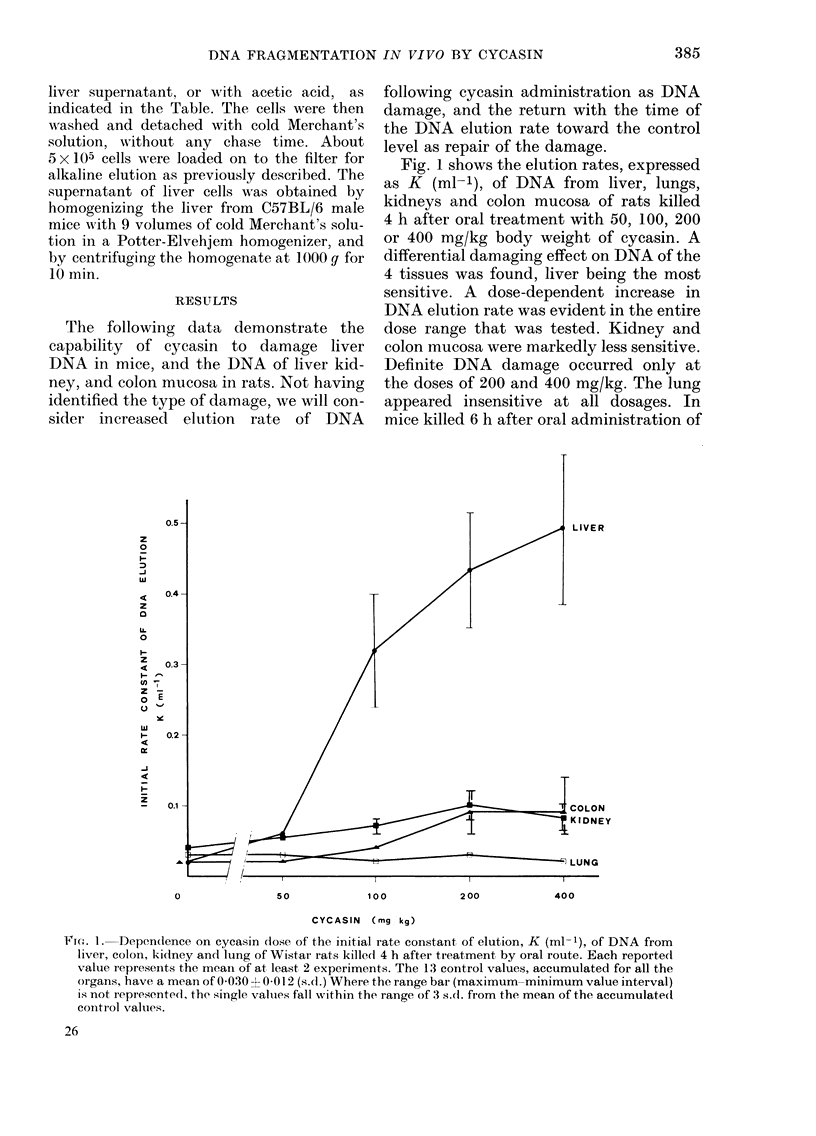
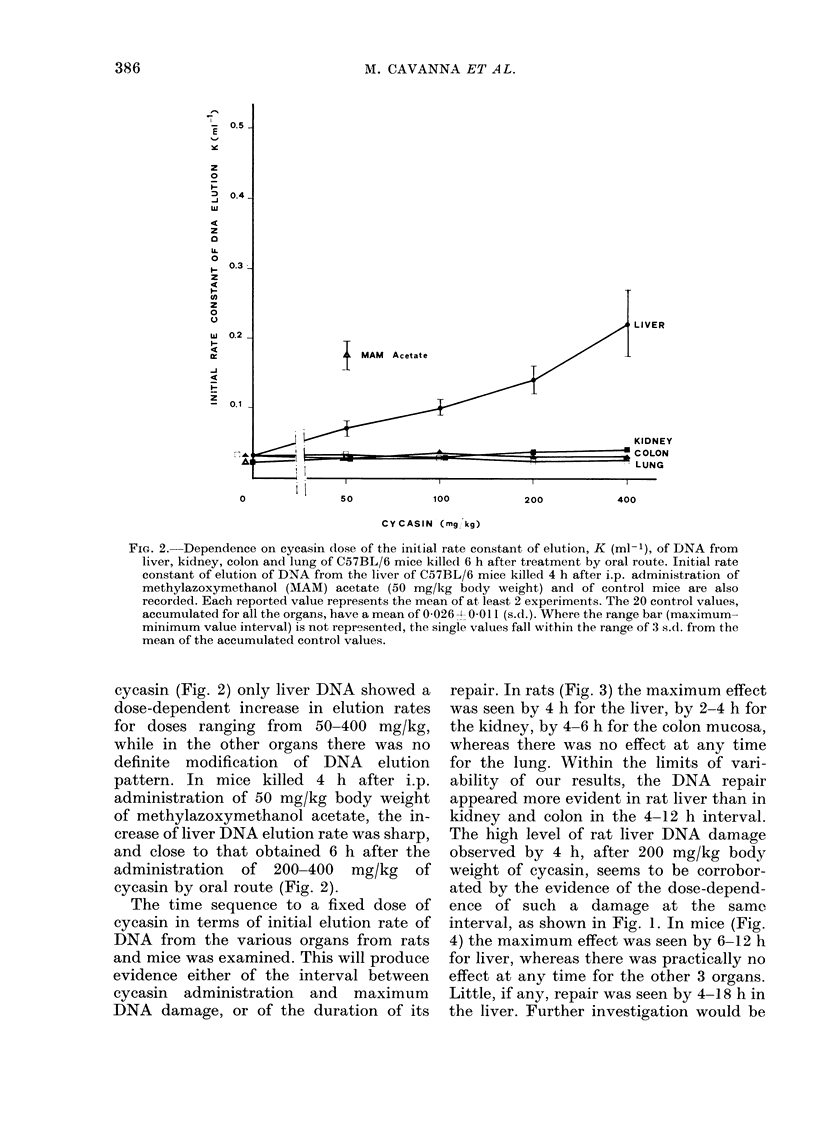
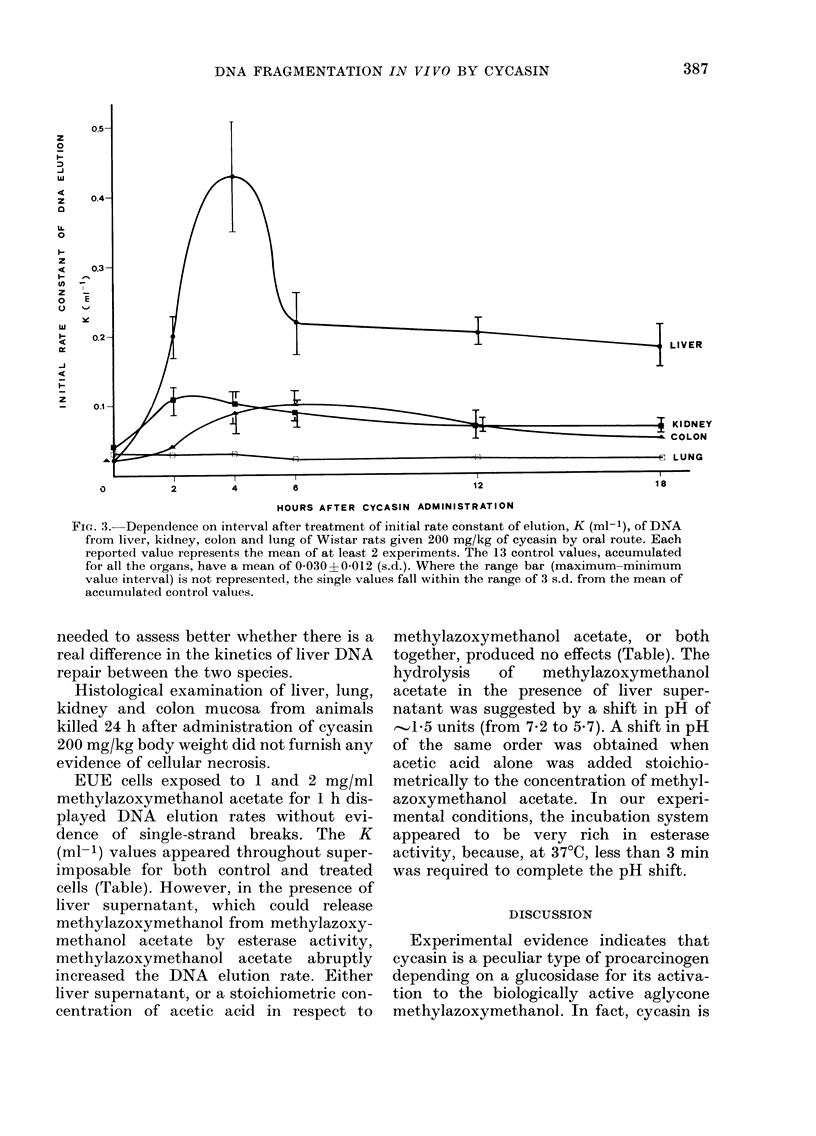
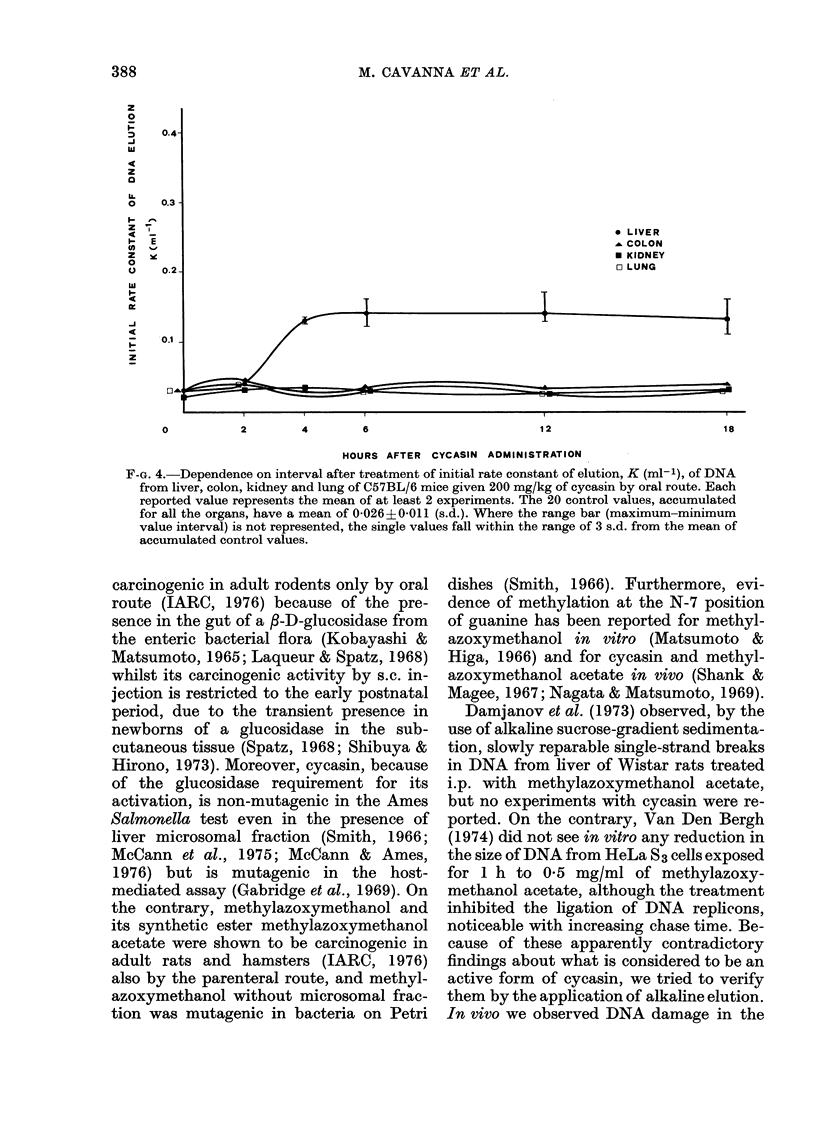


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brambilla G., Cavanna M., Parodi S., Sciaba L., Pino A., Robbiano L. DNA damage in liver, colon, stomach, lung and kidney of BALB/c mice treated with 1,2-dimethylhydrazine. Int J Cancer. 1978 Aug 15;22(2):174–180. doi: 10.1002/ijc.2910220211. [DOI] [PubMed] [Google Scholar]
- Brambilla G., Cavanna M., Sciabà L., Carlo P., Parodi S., Taningher M. A procedure for the assay of DNA damage in mammalian cells by alkaline elution and microfluorometric DNA determination. Ital J Biochem. 1977 Nov-Dec;26(6):419–427. [PubMed] [Google Scholar]
- Damjanov I., Cox R., Sarma D. S., Farber E. Patterns of damage and repair of liver DNA induced by carcinogenic methylating agents in vivo. Cancer Res. 1973 Sep;33(9):2122–2128. [PubMed] [Google Scholar]
- Gabridge M. G., Denunzio A., Legator M. S. Cycasin: detection of associated mutagenic activity in vivo. Science. 1969 Feb 14;163(3868):689–691. doi: 10.1126/science.163.3868.689. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- KOBAYASHI A., MATSUMOTO H. STUDIES ON METHYLAZOXYMETHANOL, THE AGLYCONE OF CYCASIN. ISOLATION, BIOLOGICAL, AND CHEMICAL PROPERTIES. Arch Biochem Biophys. 1965 May;110:373–380. doi: 10.1016/0003-9861(65)90137-2. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Erickson L. C., Ewig R. A., Friedman C. A. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976 Oct 19;15(21):4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- Laqueur G. L., Spatz M. Toxicology of cycasin. Cancer Res. 1968 Nov;28(11):2262–2267. [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Matsumoto H. Studies on methylazoxymethanol: methylation of nucleic acids in the fetal rat brain. Proc Soc Exp Biol Med. 1969 Oct;132(1):383–385. doi: 10.3181/00379727-132-34220. [DOI] [PubMed] [Google Scholar]
- Parodi S., Taningher M., Santi L., Cavanna M., Sciaba L., Maura A., Brambilla G. A practical procedure for testing DNA damage in vivo, proposed for a pre-screening of chemical carcinogens. Mutat Res. 1978 Aug;54(1):39–46. doi: 10.1016/0165-1161(78)90133-4. [DOI] [PubMed] [Google Scholar]
- Shank R. C., Magee P. N. Similarities between the biochemical actions of cycasin and dimethylnitrosamine. Biochem J. 1967 Nov;105(2):521–527. doi: 10.1042/bj1050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya C., Hirono I. Relations between postnatal days of mice and carcinogenic effect of cycasin. Gan. 1973 Feb;64(1):109–110. [PubMed] [Google Scholar]
- TERNI M., LO MONACO G. B. Coltura continua di cellule derivate da embrione umano. Sperimentale. 1958 May-Jun;108(3):177–185. [PubMed] [Google Scholar]
- van den Berg H. W. Alkaline sucrose gradient sedimentation studies of DNA from HeLa S3 cells exposed to methyl methanesulphonate or methylazoxymethanol acetate. Biochim Biophys Acta. 1974 Jun 27;353(2):215–226. doi: 10.1016/0005-2787(74)90186-5. [DOI] [PubMed] [Google Scholar]


