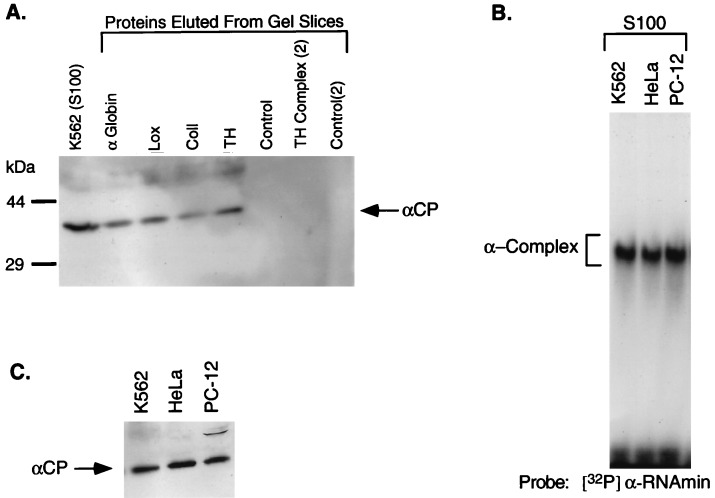
Figure 4.
αCP is a component of the RNP complexes formed on 3′UTR segments of each of four stable mRNAs. (A) RNP complexes were formed on each of the four 3′UTR segments in vitro, resolved by nondenaturing PAGE (as in Fig. 3B), and gel slices containing each of the RNP complexes were then excised. Proteins were eluted from each gel slice, concentrated by acetone precipitation, resolved by SDS/12.5% PAGE, and electrotransferred to a nitrocellulose membrane. The blot was incubated with a chicken anti-αCP antibody, and immune complexes were visualized with a secondary antibody using ECL (Amersham). The identity of each sample is shown above the lanes. TH complex (2) corresponds to the more slowly migrating of the two tyrosine hydroxylase complexes. As controls, proteins were eluted from positions on a lane containing S100 extract (no added RNA) corresponding to each RNP complex (Control) as well as from a region of the lane corresponding to the upper TH RNP complex [Control (2)]. (B) RNP complexes were formed on the α-globin 3′UTR segment (αRNAmin) with S100 extracts from three different cell types: K562, HeLa, and PC-12. The resultant complexes were electrophoresed as in Fig. 1. The identity of each S100 extract is indicated above the lanes. (C) Western blot analysis of S100 extracts from K562, HeLa, and PC-12 cells. The immunoreactive αCP is indicated.