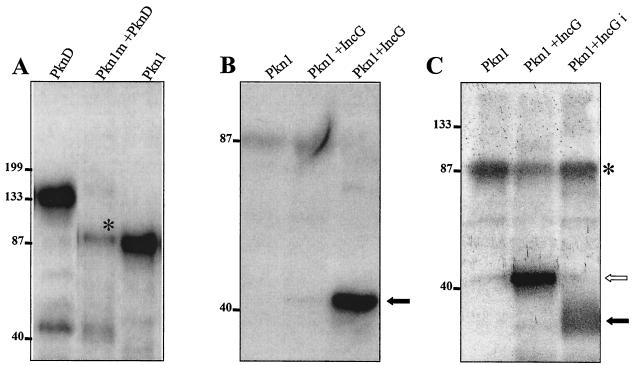
FIG. 6.
Phosphorylation of interacting partners of Pkn1. (A) E. coli-expressed and purified mutant GST-Pkn1 [K→A] and GST-PknD were used in an in vitro kinase assay. Both kinases were eluted from glutathione Sepharose beads by reduced glutathione, and the eluted proteins were used in the kinase assay. A band of Pkn1 marked with * indicates the phosphorylation of mutant Pkn1 by PknD. A faint band of lower-molecular-mass phosphorylated PknD is visible in the same lane. An autophosphorylated GST-Pkn1 bound to glutathione beads is also shown in the panel as a positive control. (B) Phosphorylation of E. coli-expressed and glutathione-eluted GST-IncG with the GST-Pkn1. A very faint phosphorylation product could be seen in the lane when reduced glutathione-eluted Pkn1 and IncG were used in the kinase reaction, whereas a solid arrow represents a strong phosphorylated product when Pkn1 bound to GST beads was used with the glutathione-eluted IncG. (C) Pkn1-mediated phosphorylation of immunoprecipitated IncG from C. trachomatis total lysates in an in vitro kinase reaction. The open arrow represents phosphorylated GST-IncG, whereas the solid arrow represents IncGi (immunoprecipitated with anti-IncG antibody from chlamydial lysates). *, the residual autophosphorylating activity of Pkn1.