Abstract
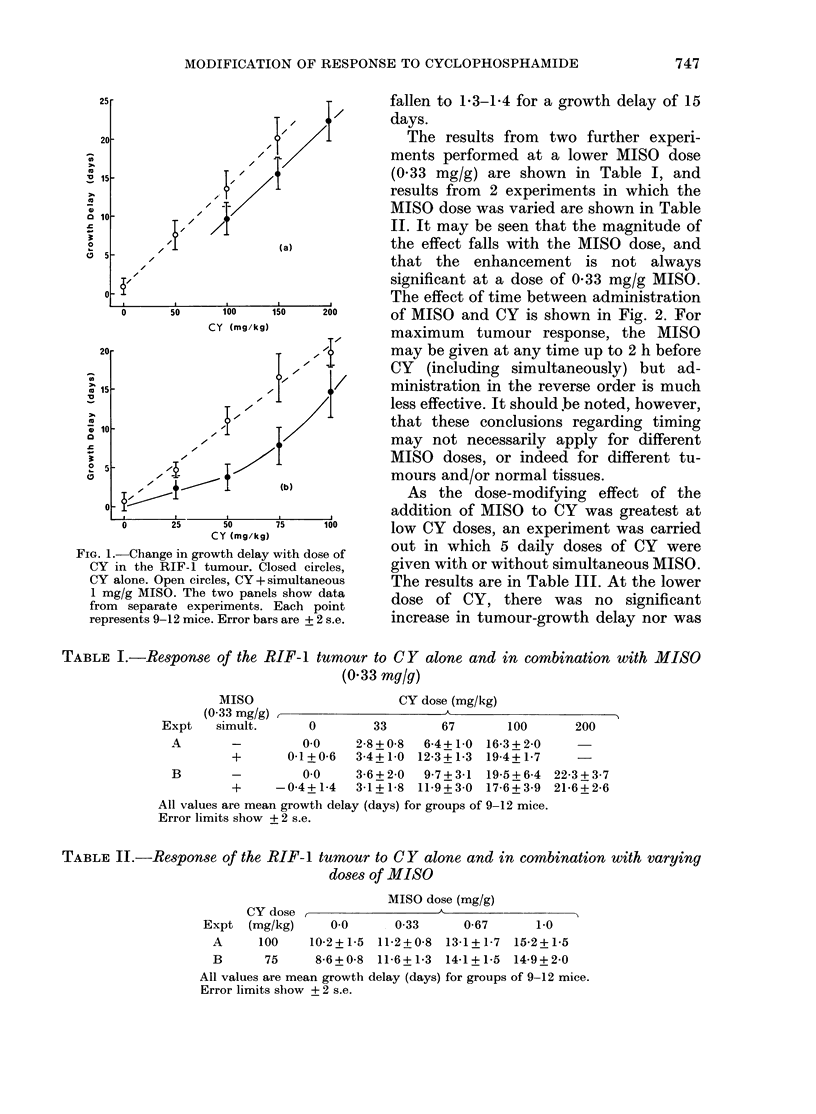
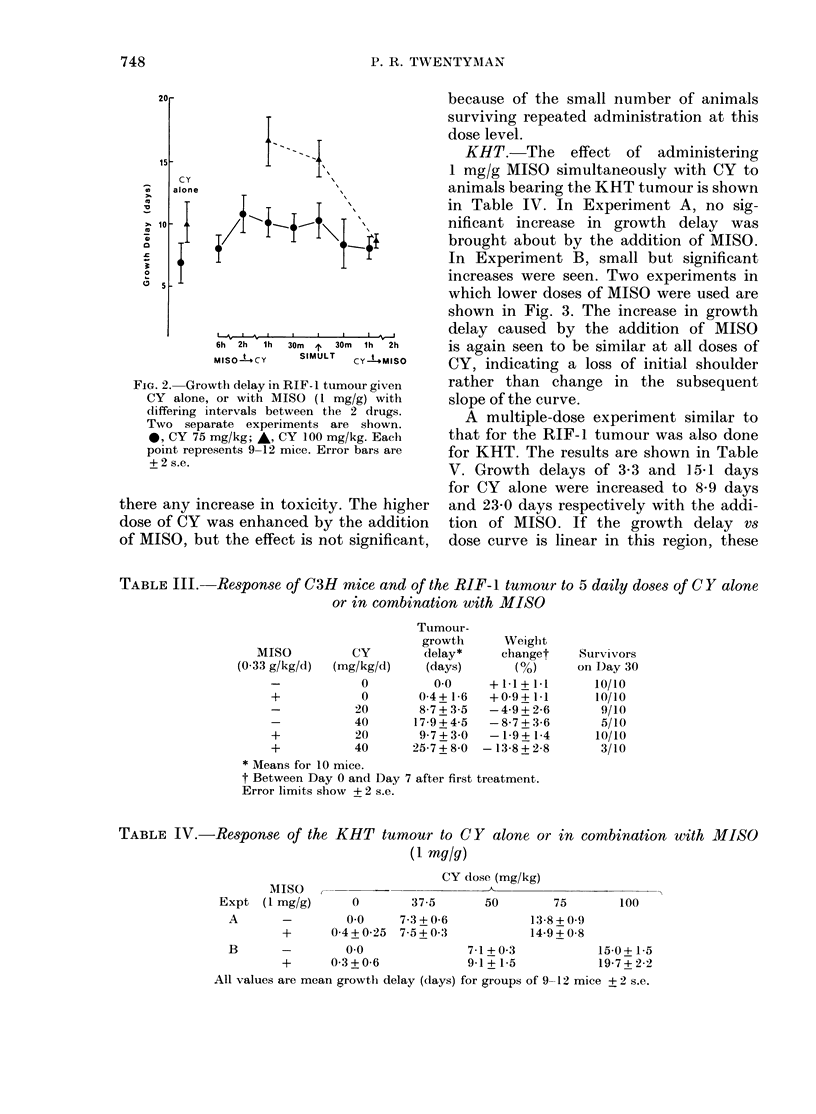
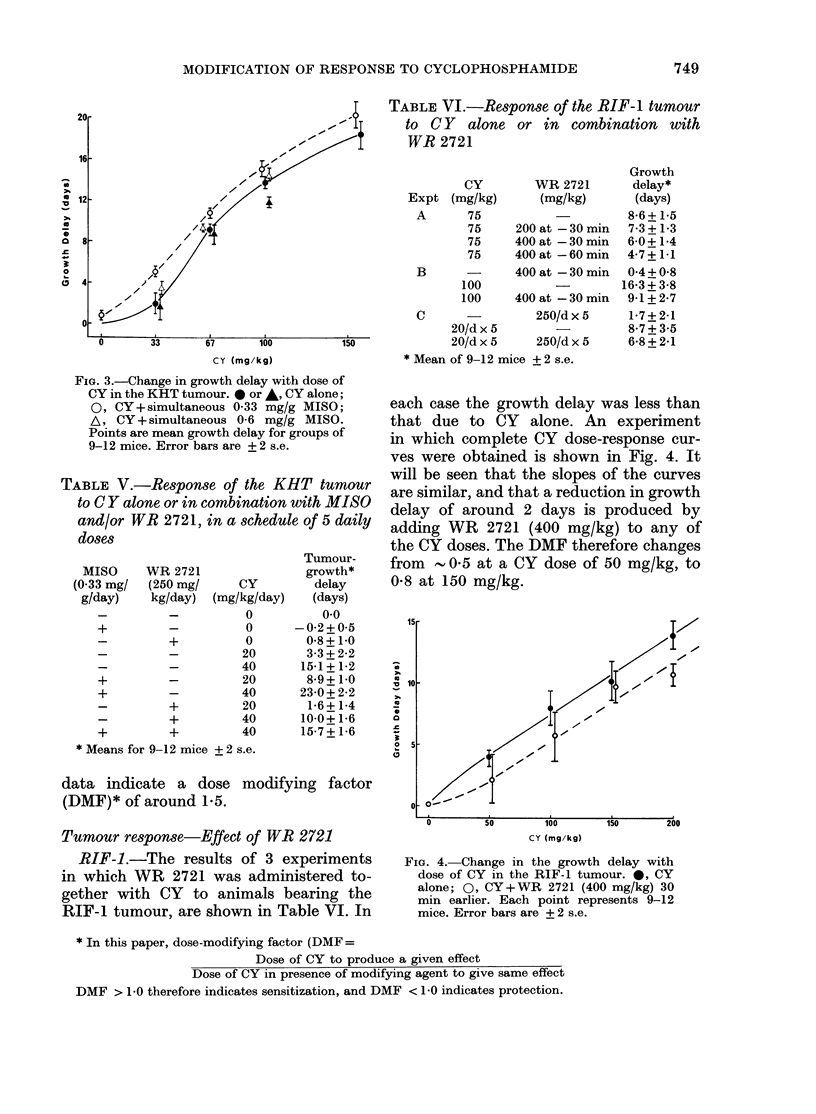
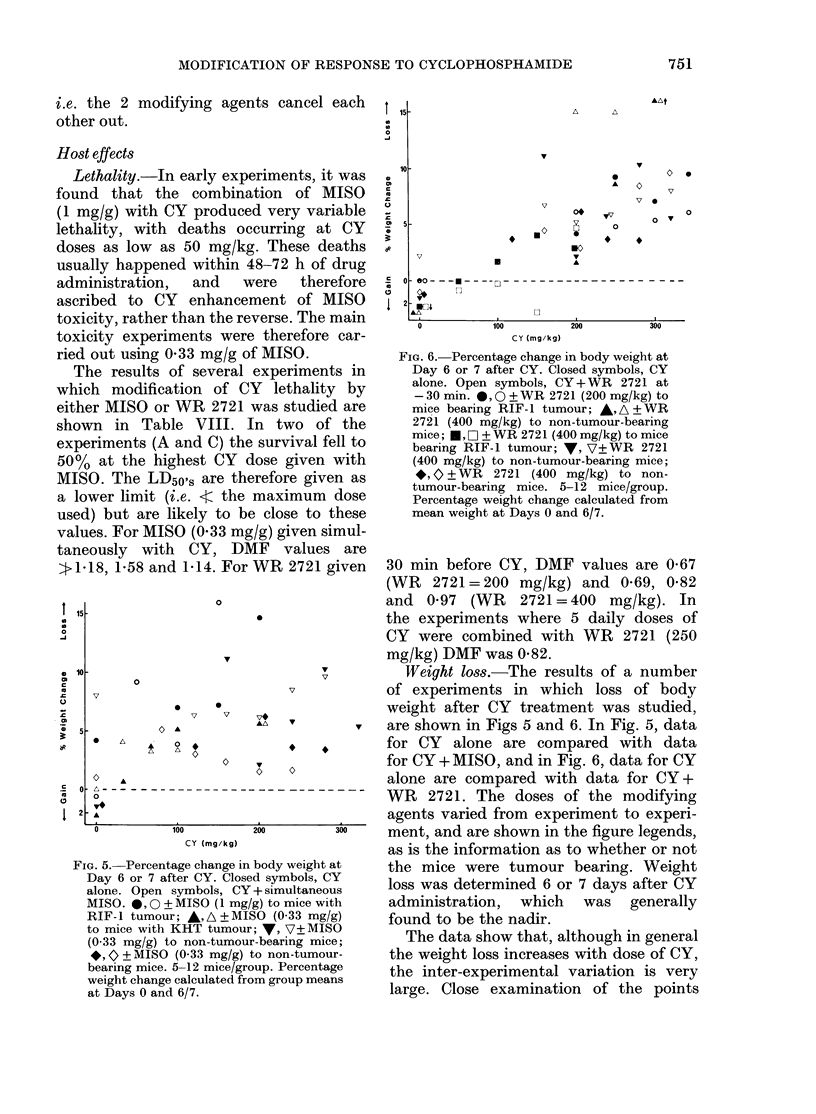
The effect has been studied of adding either misonidazole (MISO) or the radioprotective drug, WR 2721, to cyclophosphamide (CY) treatment of mice bearing either the RIF-1 or KHT sarcomas. In RIF-1, the growth delay due to CY was increased by the addition of 1 mg/g of MISO. At doses below 75 mg/kg of CY, the effect was dose modifying but, at higher doses, the curves were parallel. When the MISO dose was reduced to 0.33 mg/g, the effect was reduced but not entirely lost. Only a small enhancement of CY response in the KHT tumour was seen with single doses, but the enhancement was greater with fractionated doses. The growth delay produced by CY in both tumour systems was reduced if WR 2721 (400 mg/kg) was given 30 min earlier. At a CY dose of 75-100 mg/kg the dose-modifying factor (DMF) was approximately 0.7-0.8 but, at least in the RIF-1 tumour, was not so low at higher doses of CY. Determination of the LD50 for CY showed a DMF of approximately 1.2-1.3 for MISO (0.33 mg/g) and approximately 0.8 for WR 2721 (400 mg/kg). Neither modifying agent appeared to cause any consistent change in the pattern of body-weight loss after CY, but WR 2721 reduced the myelosuppression seen at 3-4 days after CY. The data suggest that modification of tumour response to CY by the addition of MISO varies from tumour to tumour, and is very dependent upon the MISO dose. The protective effect of WR 2721 when combined with CY is not confined to normal tissues, and at a dose of 400 mg/kg may be as great in terms of tumour response as in terms of acute LD50 in this system. At a lower dose of WR 2721, however, some differential protection may occur.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M. Cytotoxic effects of the hypoxic cell radiosensitizer Ro 7-0582 to tumor cells in vivo. Radiat Res. 1977 Dec;72(3):469–486. [PubMed] [Google Scholar]
- Brown J. M., Twentyman P. R., Zamvil S. S. Response to the RIF-1 tumor in vitro and in C3H/Km mice to X-radiation (cell survival, regrowth delay, and tumor control), chemotherapeutic agents, and activated macrophages. J Natl Cancer Inst. 1980 Mar;64(3):605–611. [PubMed] [Google Scholar]
- Clement J. J., Gorman M. S., Wodinsky I., Catane R., Johnson R. K. Enhancement of antitumor activity of alkylating agents by the radiation sensitizer misonidazole. Cancer Res. 1980 Nov;40(11):4165–4172. [PubMed] [Google Scholar]
- Gomer C. J., Johnson R. J. Relationship between misonidazole toxicity and core temperature in C3H mice. Radiat Res. 1979 May;78(2):329–333. [PubMed] [Google Scholar]
- Hall E. J., Roizin-Towle L. Hypoxic sensitizers: radiobiological studies at the cellular level. Radiology. 1975 Nov;117(2):453–457. doi: 10.1148/117.2.453. [DOI] [PubMed] [Google Scholar]
- Kallman R. F., Silini G., Van Putten L. M. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967 Sep;39(3):539–549. [PubMed] [Google Scholar]
- Stratford I. J., Adams G. E., Horsman M. R., Kandaiya S., Rajaratnam S., Smith E., Williamson C. The interaction of misonidazole with radiation, chemotherapeutic agents, or heat: a preliminary report. Cancer Clin Trials. 1980 Fall;3(3):231–236. [PubMed] [Google Scholar]
- Tannock I. F. In vivo interaction of anti-cancer drugs with misonidazole or metronidazole: cyclophosphamide and BCNU. Br J Cancer. 1980 Dec;42(6):871–880. doi: 10.1038/bjc.1980.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Twentyman P. R., Kallman R. F., Brown J. M. The effect of time between X-irradiation and chemotherapy on the growth of three solid mouse tumors--I. Adriamycin. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1255–1260. doi: 10.1016/0360-3016(79)90649-7. [DOI] [PubMed] [Google Scholar]
- Van Putten L. M., Kallman R. F. Oxygenation status of a transplantable tumor during fractionated radiation therapy. J Natl Cancer Inst. 1968 Mar;40(3):441–451. [PubMed] [Google Scholar]
- Wasserman T. H., Phillips T. L., Ross G., Kane L. J. Differential protection against cytotoxic chemotherapeutic effects on bone marrow CFUs by Wr-2721. Cancer Clin Trials. 1981;4(1):3–6. [PubMed] [Google Scholar]
- Yuhas J. M. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980 May;40(5):1519–1524. [PubMed] [Google Scholar]
- Yuhas J. M., Culo F. Selective inhibition of the nephrotoxicity of cis-dichlorodiammineplatinum(II) by WR-2721 without altering its antitumor properties. Cancer Treat Rep. 1980 Jan;64(1):57–64. [PubMed] [Google Scholar]
- Yuhas J. M. Differential protection of normal and malignant tissues against the cytotoxic effects of mechlorethamine. Cancer Treat Rep. 1979 Jun;63(6):971–976. [PubMed] [Google Scholar]


