Abstract
Streptococcus pneumoniae meningitis remains a disease with a poor outcome for the patient. A region of the brain that has been neglected in the study of meningitis is the ependyma, which has been identified as a location of adult pluripotent cells. In this study we have used a rat model of meningitis to examine whether the ependymal layer is affected by S. pneumoniae. The effects included localized loss of cilia, a decrease of the overall ependymal ciliary beat frequency, and damage to the ependymal ultrastructure during meningitis. In conclusion, loss of ependymal cells and ciliary function exposes the underlying neuronal milieu to host and bacterial cytotoxins and this is likely to contribute to the neuropathology commonly observed in pneumococcal meningitis.
Despite modern intensive care, antibiotic treatment, and the use of dexamethasone, the morbidity and mortality associated with Streptococcus pneumoniae meningitis remain high (15). Vaccines against this bacterium show no evidence of reducing the number of cases (17). The mechanisms by which S. pneumoniae causes neuronal damage (once it infects the cerebrospinal fluid [CSF]) are yet to be fully deciphered. To investigate the effect of S. pneumoniae and its virulence factors, we established an ex vivo method for the culture and differentiation of brain tissue into a monolayer of ciliated ependymal cells. Ependymal cells line the ventricular cavities and the cerebral aqueduct separating the CSF that is infected in meningitis and ventriculitis from neuronal tissue. Using this model, investigators have previously shown that both pneumolysin (4, 12) and pneumococcal hydrogen peroxide (H2O2) (5) cause damage to ependymal cells. In addition, when clinically relevant concentrations of bacteria were added to brain slices prepared from the fourth ventricle there was a rapid inhibition of ependymal ciliary beat frequency (CBF) (5).
The CBF of ependymal cilia may be measured directly and continually to assess the functioning and integrity of ependymal cells. The ependyma is thought to act as a filter relaying macromolecules to and from the CSF, and it also plays a role in controlling CSF volume (3). Recently, both ependymal (8) and subventricular zone (1) cells have been identified as adult neuronal stem cells, stressing the potential importance of the thin cellular layer that lines the ventricles and aqueducts of the brain.
It was essential to determine whether our ex vivo findings (determined using the ciliated ependymal cells) were also observed during pneumococcal meningitis. We therefore established a modification of the rat meningitis model described by Pfister and colleagues (14). In this paper we show that during pneumococcal meningitis and as predicted from our ex vivo studies, ependymal CBF is inhibited and the integrity of the ependymal layer is compromised.
Overview.
A cannula was surgically implanted onto the atlanto-occipital membrane prior to perforation, according to the method of Huang and colleagues (6). The CSF was then infected with the desired bacterial dose. The cannula allowed access to the CSF at all times following infection, and we were able to take samples from a conscious rat with the minimal amount of trauma to the animal and its brain tissue.
Surgical implantation of cisterna cannula.
All materials used throughout this study were of the utmost purity, and all surgical conditions and instruments were aseptic throughout. Male Wistar rats (Harlan, Bicester, United Kingdom) (300 to 400 g) were anesthetized by halothane (3 to 5% [vol/vol] for induction and 2% [vol/vol] for maintenance), and their heads were then shaved with small-animal clippers. The rats were administered Temgesic (0.1 ml/kg of body weight) subcutaneously and mounted in a stereotaxic frame with the nose bar at the full caudal position so that the rat's head was postured at an acute downward angle, thus allowing extension of the atlanto-occipital membrane. Once the head was secure, the skin was swabbed with Hibitane in alcohol prior to a midline incision (2 to 3 cm long) through the skin to expose the skull and the dorsal neck muscles. The subcutaneous membrane on the skull was removed (using cotton bud sticks) by gentle abrasion. A midline incision was then made through the dorsal neck muscles between the top of the occipital bone and the atlas arc. The ligatures attaching the muscle to the occipital bone were then cut, and blunt dissection was employed to reveal the atlanto-occipital membrane covering the cisterna magna. A 19-gauge small-animal cannula (Plastics One, Roanoke, Va.) (13 mm long) with a dummy cannula insert was then glued (using Histoacryl Blue; B. Braun Medical Ltd., Sheffield, United Kingdom) to the atlanto-occipital membrane. The cannula was secured to the skull by a dental cement (Aquacem; Dentsply, De-Trey, Constanz, Germany) cast. Once the liquid dental cement had cured, the wound was sutured and the cannula was further secured with a blanket stitch. The dummy cannula was then inserted into the cannula and the rats were allowed to recover. The rats were given Temgesic (0.1 ml/kg/day) for 48 h after the surgery, weighed daily, and given a soaked diet for 7 days to allow for full recovery from the procedure.
Intracisternal infection.
The rats were anesthetized (3% [vol/vol] halothane) and mounted on the stereotaxic frame as described above. The dummy cannula was removed from the cannula, and a total of 104 CFU of S. pneumoniae in 50 μl of phosphate-buffered saline (PBS) (pH 7.4) was administered intracisternally using a gas-tight syringe (SGE; Fisher Scientific, Loughborough, United Kingdom). The location of the needle in the cisterna magna was confirmed by a backflow of clear CSF into the syringe. Once the dose was assimilated (2 min), the needle was removed and the dummy cannula was replaced. The animal was then allowed to recover and was observed every 3 to 6 h for signs of the development of clinical symptoms. When the rat was observed to be 2+ lethargic (minimal movement) (13), the experiment was terminated. The meningitis group contained 16 rats, and the control group (consisting of rats that were administered intracisternal injections of 50 μl of sterile PBS) contained 11 rats.
CSF sampling.
At the end of the experiment, the rats were given a lethal intraperitoneal injection of sodium pentobarbitone (Euthatal [3 ml]). Approximately 50 μl of the cisternal CSF was sampled (using a gas-tight syringe [SGE; Fisher Scientific]) through the cannula as described above. Blood was taken by cardiac puncture, and lungs and brains were also dissected and washed in 2 × 10 ml of PBS prior to homogenization.
Tissue processing.
The CSF was used for bacterial colony and white cell counting and lactate and glucose measurements. The blood was only used for bacterial colony counting. The right prefrontal cortex and olfactory bulb of the brain and the lungs were homogenized (using an Ultraturax apparatus; IKA Labortechnik, Staufen, Germany) (5 × 30-s bursts in 10 ml of sterile PBS) and used for bacterial colony counting. The cerebellum or the midbrain was dissected for CBF measurements of the fourth and lateral ventricles.
CBF measurements.
The method used for this study was similar to a method previously described (4). Unfixed fresh sections (250 μm) were cut (using a vibratome) into the floor of the fourth and left lateral ventricles. The brain slices were placed in 3 ml of HEPES (25 mM)-buffered medium 199 (pH 7.4) in a humidified (80 to 90%) thermostatically controlled incubation (37°C) chamber surrounding a light microscope (Diphot; Nikon, Kingston on Thames, Surrey, United Kingdom) and left to equilibrate for 30 min. Beating cilia were recorded (×400 magnification) using a digital high-speed video camera (Kodak Ektapro Motion Analyser, model 1012) at a rate of 400 frames per s and a shutter speed of 1/2,000 s. The camera allowed video sequences to be recorded and played back at reduced frame rates or by individual frames. The CBF was determined by timing a given number of individual cilium beat cycles. After 60 min of equilibration, the final readings were taken. The ciliated edges of the ventricles were separated into zones. Zone 1 was the left wall (100 μm from the ventricle floor), zone 2 was the floor, and zone 3 was the right wall (100 μm from the ventricle floor) of both the fourth and lateral ventricles. Three readings were taken (using two to four serial slices per rat) from each zone at equidistant points. In cases in which there was a loss of cilia on the cells for which the three readings were taken within each zone, this loss was noted and the total number of lost cilia was expressed as a percentage of loss (i.e., 100% represents a fully ciliated ependyma). In total, between 18 and 36 CBF or cilium measurements per brain were taken.
Bacteria.
S. pneumoniae strain D39, serotype 2, was used. To standardize virulence measurements, all bacteria were passaged through the mouse prior to use in the meningitis model (9). Bacteria were grown in brain heart infusion broth to late log phase (optical density at 500 nm, 0.6 to 0.8), and bacterial suspensions were frozen (−80°C) in brain heart infusion broth containing 20% (vol/vol) fetal calf serum. Thawed bacteria were sedimented (6,000 rpm for 2 min in a microcenteur apparatus [Heraeus, Hanau, Germany]), resuspended in PBS at the required concentration, and kept on ice for no more than 30 min before being used as the infective dose.
Bacterial colony counting.
Bacteria were counted as previously described (5).
CSF parameters.
For glucose experiments, 5 μl of test CSF was added to 1.0 ml of glucose reagent (Trinder; Sigma Diagnostics, Poole, United Kingdom) containing 4-aminoantipyrine (0.5 mM), p-hydroxybenzene sulfonate (20 mM), glucose oxidase (15,000 U/liter), and horseradish peroxidase (10,000 U/liter) at pH 7.0. For positive controls, standard CSF samples (Sigma Diagnostics) were assayed alongside the CSF from rats. The samples were incubated at 37°C for 10 min, and the glucose concentration was then calculated from the A500 value.
Lactate.
A total of 10 μl of test CSF was added to 1.0 ml of lactate reagent (Sigma Diagnostics)-lactate oxidase (400 units/liter)-horseradish peroxidase (2,400 U/liter) buffered (with Tris-HCl) at pH 7.2. Standard CSF (Sigma Diagnostics) was assayed in parallel. The samples were incubated at room temperature for approximately 10 min, and the lactate concentration was calculated from the A540 value.
CSF white cell counting.
Cells were counted in either Fuchs-Rosenthal (for samples of up to 103 cells/ml) or hemocytometer (for samples above 103 cells/ml) counting chambers. The cells in the CSF (20 μl) were fixed with methanol (20 μl) for 30 min prior to addition of Giemsa stain (Fisher Chemicals) (20 μl; 1-in-4 dilution in filter-sterilized PBS). The counting was performed after a 10-min incubation at room temperature, and individual white cells were identified (using light microscopy) according to their morphological characteristics.
Electron microscopy.
For scanning electron microscopy (SEM; ISI, Santa Barbara, Calif.) performed at 15 kV, the tissues were fixed in Sorensen's phosphate-buffered glutaraldehyde (Sigma Diagnostics) (pH 7.4; 4% [wt/vol] for 48 h). After postfixation in 1% [wt/vol] osmium tetroxide, samples were dehydrated through ethanol gradients and immersed in hexamethyldisilazane. The hexamethyldisilazane was evaporated, leaving dry tissue with no phase boundary damage. The SEM specimens were sputter coated with gold. For transmission electron microscopy (TEM) (JEOL, Tokyo, Japan), thin (70 nm) sections of the ependyma were cut using a glass blade microtome (Ultracut E; Reichert, Vienna, Austria).
Statistics.
Data are presented as means ± standard errors of the means of 5 to 16 independent experiments. Statistical analysis was performed (where appropriate) using GraphPad Prism, version 2. Data were compared using parametric (paired or unpaired) Student's t tests and were considered significant when P values were <0.05.
Impact of surgery.
During surgery, there were no adverse incidents in the anesthetic regimen or the procedure. On the day of surgery, rats in the control and meningitis groups weighed between 312 and 382 g. On the day of infection (7 days later), the weights for the control and meningitis rats ranged between 285 and 363 g. The total weight loss before infection amounted to approximately 10% of initial body weights, and all rats were regaining body weight at the time of infection (day 7).
Disease.
Following infection, rats in the meningitis group showed some symptoms (1+ starry coat [defined as slight piloerection]) by 6 h and by 26.2 ± 1.4 h all had reached the stage that was the endpoint of the experiment (2+ lethargic) and were humanely euthanized. The control group showed no symptoms throughout the 48 h following PBS inoculation.
Bacterial counts.
Meningitis was confirmed by a positive bacterial count in the CSF (Table 1) and also by significantly (P < 0.05) elevated total white cell CSF counts (see below). Of the rats with meningitis, 10 also developed bacteremia, 11 had pneumococcal invasion of brain tissue, and 2 had pneumococcal colonization of the lungs (Table 1). All cisternally infected rats had CSF colony counts above those found in other tissues.
TABLE 1.
Bacterial counts in tissue, blood, and CSFa
| Tissue or fluid | Resultsb | No. of rats infected/ total no. (%) |
|---|---|---|
| Fluids | ||
| CSF | 7.3 ± 0.4c log CFU/ml | 16/16 (100) |
| Blood | 5.3 ± 0.4c log CFU/ml | 10/16 (62.5) |
| Tissues | ||
| Brain | 4.7 ± 0.2c log CFU/mg | 11/16 (68.7) |
| Lung | 4.0 log CFU/mg | 2/16 (12.5) |
Samples from control group rats showed no bacterial counts for tested strains.
Data, expressed no means ± standard errors of the means, represent bacterial counts for 16 meningitis group rats.
Significantly (P < 0.05) increased compared to control group.
CSF biochemistry.
In infected animals, CSF lactate levels were slightly elevated (8 ± 2 mmol/liter for the control group and 10 ± 1 mmol/liter for the meningitis group) and glucose levels were reduced (44 ± 18 mmol/liter for the control group and 28 ± 6 mmol/liter for the meningitis group).
CSF white cells.
Analysis of the immune cells within the CSF of control and meningitis rats showed that leukocyte levels were significantly (P < 0.05) elevated in the meningitis group (194 ± 85 cells/μl) compared to those observed for the control group (6.1 ± 1.1 cells/μl).
Ependymal CBF.
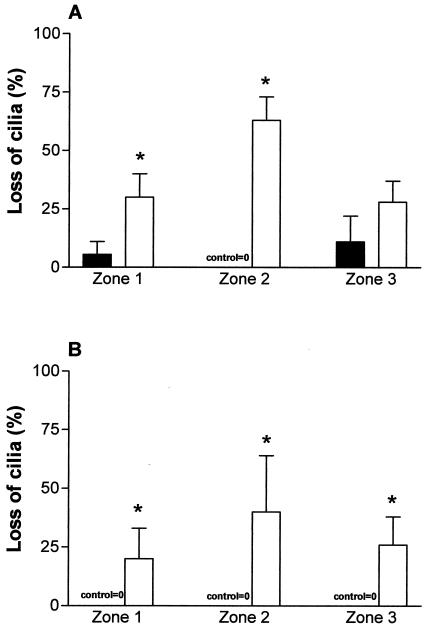
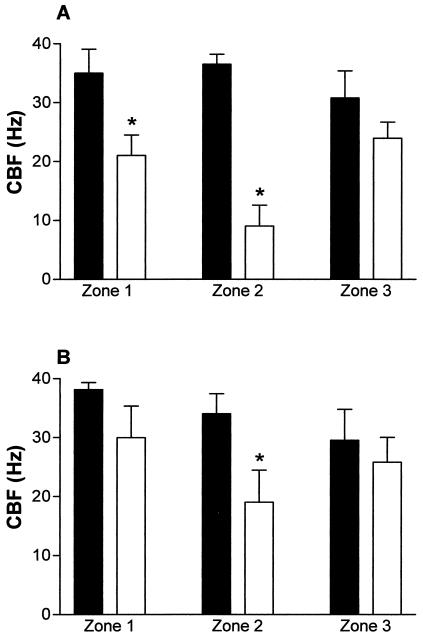
In meningitis group rat brains, the mean ependymal CBF in all zones from both ventricles (20.2 ± 2 Hz) was significantly (P < 0.05) reduced compared to that observed with control group rat brains (33.6 ± 1.5 Hz). An obvious difference between the control ependyma and the meningitis ependyma was the loss of cilia from all of the zones in the meningitis group. Zone 2 was the most denuded of cilia in both ventricles (Fig. 1). In zones 1 and 2 of the fourth ventricle of meningitis group mice, the number of cilia was significantly (P < 0.05) decreased compared to that seen with the control mice (Fig. 1A). In the meningitis group, there was also a significant (P < 0.05) loss of cilia in the lateral ventricle (Fig. 1B) compared to that seen with the control group. The effect of the loss of cilia on the mean overall CBF in the meningitis group is shown in Fig. 2. In the meningitis group (Fig. 2), there was a significant (P < 0.05) decrease in the CBF in zone 2 from both ventricles compared with that seen with the control group. In addition, zone 1 (left wall) of the fourth ventricle of rats with meningitis also had a significant (P < 0.05) decrease of CBF compared with that seen with control rats (Fig. 2A). There was no significant difference between the fourth and lateral ventricles in control CBF.
FIG. 1.
Loss of cilia in the fourth (A) and lateral (B) ventricles of control rats (closed bars) and rats with meningitis (open bars). Zones with no cilia were recorded, and the data represent the number of cilium-free observations expressed as a percentage of the total number of observations. A result of 0% represents a fully ciliated ependyma. *, statistically (P < 0.05) significant compared to control.
FIG. 2.
Measurement of brain fourth (A) and left lateral (B) ventricular functional CBF in control rats (closed bars) and rats infected with 104 CFU of S. pneumoniae (open bars). *, the CBF in the floor of the ventricles (zone 2 [A and B] and zone 1 [A]) was significantly (P < 0.05) inhibited compared to that seen with control group rats.
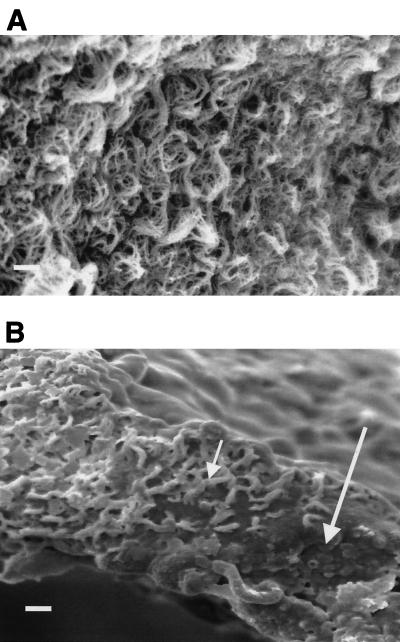
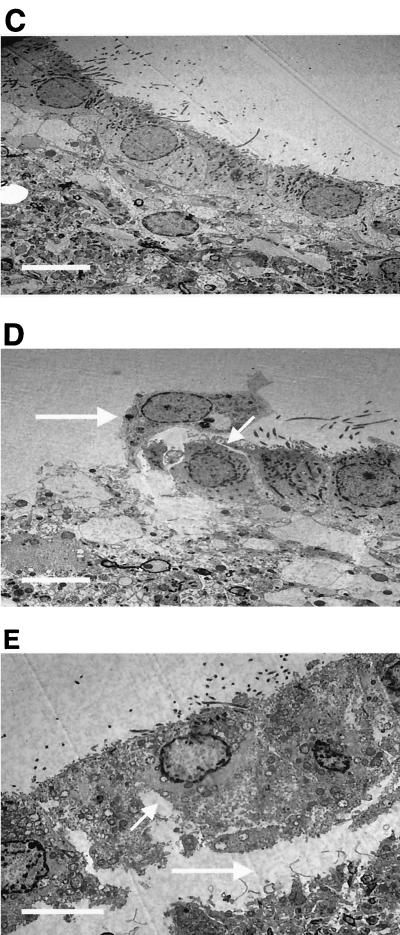
Qualitative ultrastructure.
SEM of control tissue showed a healthy fully ciliated epithelium (Fig. 3A). This was in contrast to the evidence of ependymal disruption during meningitis (Fig. 3B). This picture (Fig. 3B) clearly shows that in contrast to control results (Fig. 3A), there is a loss of cilia from the zone around the floor of the fourth ventricle and a tendency for the remaining cilia to stick together. TEM of the ventricle floor (zone 2) in the overall tissue as well as in the individual cells of the brain epithelium in rats with meningitis (Fig. 3D and E) also demonstrated a lack of integrity compared to that of control group rats (Fig. 3C). The micrographs showed individual ependymal cell detachment (Fig. 3D) and ependymal cell layer detachment (Fig. 3E) from the underlying cellular layer. In addition, in the micrographs from the meningitis rats, large openings of the gap junctions between ependymal cells were visible. Due to thorough washing prior to electron microscopy analysis, bacteria or associated white cells were not evident on the ependymal sections.
FIG. 3.
SEM (A and B) and TEM (C, D, and E) results for the floor (zone 2) of the fourth ventricle for control rats (A and C) and rats with meningitis (B, D, and E) (CSF at 107 CFU/ml). The small arrow in panel B shows clumping of the cilia, and the large arrow in panel B shows the loss of cilia in the floor of the fourth ventricle, with no cilia present. The TEM images show the opening of gap junctions (D and E; small arrows) and detachment of the ependymal cells (D and E; large arrows). Images are representative of 50 others. Bar, 10 μm.
After an inoculation of 104 CFU was performed, we observed reproducible development of meningitis with a significant increase in CSF pneumococcal counts. Infected rats developed meningitis; the mean time for full clinical development of the disease (2+ lethargic) was 26 h. The bacteria also invaded other tissues and fluid such as blood, lungs, and brain. The apparent presence of S. pneumoniae in the grey matter of the brain may be evidence that the bacteria move from the CSF to deeper brain centers. It is possible, however, that a portion of the bacteria in these tissues was from infected blood, despite the fact that each organ was washed thoroughly with PBS prior to homogenization. The modest changes in CSF parameters that we observed (in cases in which these changes were clinically more pronounced) were probably due to the absence of an initial systemic infection prior to CSF infection in our meningitis model.
The results of this study support those found using our ex vivo ependymal model. In rats infected with S. pneumoniae, functional CBF levels were decreased and significant damage to the ependymal layer was visible by both electron and light microscopy. Using TEM we have also shown that during meningitis, the ultrastructure of the ependyma was altered. S. pneumoniae meningitis caused opening of the gap junctions between the ependymal cells, and ependymal cell detachment was apparent. The only difference between our ex vivo and in vivo findings was that as opposed to the widespread inhibition seen with our ex vivo model, in vivo the ependymal CBF was inhibited predominantly around the floor of the ventricles. This can be explained by the fact that the bacteria in the ex vivo experiments were in contact with the whole of the ependyma, whereas in vivo there may have been differences in the distribution of the bacteria between the zones.
We believe that damage to the ependymal cilia can allow pneumococci to adhere to the ependyma and accelerate ependymal damage. This would allow pneumococci, their toxins, and inflammatory mediators access to the underlying neuronal tissue of the brain, causing injury and neuronal apoptosis (2). Damage to the ependymal cells may affect brain repair, particularly as the pluripotent neural stem cells reside near the damaged ependyma in the adult brain (8). Ependymal cells do not appear to regenerate following insult, and the long-term effects of a loss of these cells are uncertain (16).
Ciliated ependymal cells densely line the ventricular cavities, aqueducts, and central canal of the spinal cord. The cilia beat rapidly, propelling CSF in the vicinity of the ependyma throughout the ventricular system. The need for such a well-developed system of moving CSF close to ependymal cells is not fully understood, but it is not important in the bulk flow of CSF (3). Movement of CSF in this area may be important in host defense, preventing debris from settling on the ependymal surface, or in facilitating diffusion of toxins from neuronal tissue to the CSF. Another potential role would be in the movement of CSF past primary cilia, which may help to regulate the CSF volume and pressure. It is thought that infection of the CSF causes resident macrophages and activated glial cells to produce chemotactic cytokines, which attract the migration of leukocytes from the periphery across the blood-brain barrier into the CSF (11). CSF flows in a predictable fashion from the choroid plexus and the lateral ventricles through the ventricular system. It is likely that the damage seen in the fourth and lateral ventricles was due to direct damage from the pneumococci; it is also possible that damage was occurring secondary to inflammation caused by invading leukocytes. It will be important to determine in future studies whether pneumococci adhere to and invade the ependymal cells and whether they invade more deeply into the neuronal tissue. The results of the elevated total leukocytes in the CSF of rats inoculated with S. pneumoniae are consistent with other reports of rat studies (10) and are characteristic of meningitis in humans (7).
We are presently investigating the various virulence factors produced by S. pneumoniae and also other factors from the host that influence the damage to the ependymal tissue. Such information will allow us to determine areas of virulence that may be targeted by adjunctive therapy.
Acknowledgments
This work was supported by a grant from the Wellcome Trust, London, United Kingdom.
We thank the members of the Division of Biomedical Services at Leicester University for their help in establishing the model of meningitis.
Editor: J. N. Weiser
REFERENCES
- 1.Alvarez-Buylla, A., D. G. Herrera, and H. Wichterle. 2000. The subventricular zone: source of neuronal precursors for brain repair 26. Prog. Brain Res. 127:1-11. [DOI] [PubMed] [Google Scholar]
- 2.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis 37. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Bigio, M. R. 1995. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia 14:1-13. [DOI] [PubMed] [Google Scholar]
- 4.Hirst, R. A., A. Rutman, K. Sikand, P. W. Andrew, T. J. Mitchell, and C. O'Callaghan. 2000. Effect of pneumolysin on rat brain ciliary function: comparison of brain slices with cultured ependymal cells. Pediatr. Res. 47:381-384. [DOI] [PubMed] [Google Scholar]
- 5.Hirst, R. A., K. S. Sikand, A. Rutman, T. J. Mitchell, P. W. Andrew, and C. O'Callaghan. 2000. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect. Immun. 68:1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, Y. L., A. Saljo, A. Suneson, and H. A. Hansson. 1995. A new approach for multiple sampling of cisternal cerebrospinal fluid in rodents with minimal trauma and inflammation 157. J. Neurosci. Methods 63:13-22. [DOI] [PubMed] [Google Scholar]
- 7.Hussein, A. S., and S. D. Shafran. 2000. Acute bacterial meningitis in adults. A 12-year review. Medicine (Baltimore) 79:360-368. [DOI] [PubMed] [Google Scholar]
- 8.Johansson, C. B., S. Momma, D. L. Clarke, M. Risling, U. Lendahl, and J. Frisen. 1999. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96:25-34. [DOI] [PubMed] [Google Scholar]
- 9.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koedel, U., and H. W. Pfister. 1999. Models of experimental bacterial meningitis. Role and limitations. Infect. Dis. Clin. N. Am. 13:549-577, vi. [DOI] [PubMed] [Google Scholar]
- 11.Leib, S. L., and M. G. Tauber. 1999. Pathogenesis of bacterial meningitis. Infect. Dis. Clin. N. Am. 13:527-548, v-vi. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed, B. J., T. J. Mitchell, P. W. Andrew, R. A. Hirst, and C. O'Callaghan. 1999. The effect of the pneumococcal toxin, pneumolysin on brain ependymal cilia. Microb. Pathog. 27:303-309. [DOI] [PubMed] [Google Scholar]
- 13.Morton, D. B., and P. H. Griffiths. 1985. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment 79. Vet. Rec. 116:431-436. [DOI] [PubMed] [Google Scholar]
- 14.Pfister, H. W., U. Koedel, U. Dirnagl, R. L. Haberl, W. Feiden, and K. M. Einhaupl. 1990. Superoxide dismutase inhibits brain oedema formation in experimental pneumococcal meningitis. Acta Neurochir. Suppl. (Vienna) 51:378-380. [DOI] [PubMed] [Google Scholar]
- 15.Quagliarello, V. J., and W. M. Scheld. 1997. Treatment of bacterial meningitis. N. Engl. J. Med. 336:708-716. [DOI] [PubMed] [Google Scholar]
- 16.Sarnat, H. B. 1995. Ependymal reactions to injury. A review. J. Neuropathol. Exp. Neurol. 54:1-15. [DOI] [PubMed] [Google Scholar]
- 17.Tan, T. Q. 2002. Prevention of pneumococcal meningitis. Curr. Infect. Dis. Rep. 4:317-323. [DOI] [PubMed] [Google Scholar]