Abstract
Syphilis has been recognized as a disease since the late 1400s, yet there is no practical vaccine available. One impediment to the development of a vaccine is the lack of understanding of multiple reinfections in humans despite the development of robust immune responses during the first episode. It has been shown that the Treponema pallidum repeat protein K (TprK) differs in seven discrete variable (V) regions in isolates and that the antibody response during infection is directed to these V regions. Immunization with TprK confers significant protection against infection with the homologous strain. We hypothesize that the antigenic diversity of TprK is involved in immune evasion, which contributes to the lack of heterologous protection. Here, using the rabbit model, we show a correlation between limited heterologous protection and tprK diversity in the challenge inoculum. We demonstrate that antibody responses to the V regions of one TprK molecule show limited cross-reactivity with heterologous TprK V regions.
Despite the fact that humans develop robust immune responses against Treponema pallidum subsp. pallidum, the etiologic agent of syphilis, humans can be infected multiple times (13). People infected with T. pallidum develop specific immune responses that are able to clear millions of treponemes from sites of primary and secondary syphilis (9); similar immune responses are seen during infection in the rabbit model (2, 12). The response is a T-cell-mediated delayed-type hypersensitivity response in which T cells infiltrate syphilitic lesions and activate macrophages to phagocytose antibody-opsonized treponemes (2, 9, 12, 20). How treponemes from heterologous isolates can evade the recall response of a previously infected individual is unknown.
It has been shown that infected rabbits develop complete immunity to challenge with the homologous isolate but that they develop less protection against heterologous isolates (19). The rabbit model used to assess protection recapitulates early human syphilis. Naive rabbits that are challenged intradermally with T. pallidum develop lesions teeming with treponemes, and these lesions progress to ulceration, much like the chancres of early syphilis. Rabbits that are protected by homologous infection do not develop lesions, inoculation sites do not support treponeme proliferation, the inoculation sites do not ulcerate, and antibody titers do not increase, indicating reinfection has not occurred (19). It is not known what immune mechanisms lead to complete homologous protection in the rabbit model and why these responses do not completely protect against heterologous challenge. Rabbits that receive passive transfers of antibodies from infection-immune rabbits and undergo intradermal homologous challenge develop delayed and altered lesions that appear after antibody administration is suspended (4). This suggests that antibodies are insufficient to eradicate T. pallidum from the host. To study the effects of T cells is more complicated. Lymphocyte transfers are not possible in the best-characterized animal model, the outbred rabbit. In the guinea pig model, in which the 50% infectious dose is considerably higher than in rabbits or humans and the clinical signs of disease are less apparent (22), adoptive T-cell transfers have prevented lesion development after homologous challenge but do not prevent infection (21). These data indicate that both antibodies and T cells play a role in protection but neither alone prevents infection. It is possible that antigenic diversity of T. pallidum accounts for the lack of heterologous protection.
The T. pallidum repeat protein K (TprK) is a strong candidate for a treponemal factor involved in immune evasion. Sequence analyses revealed that there is only one tprK locus, but there are multiple heterogeneous alleles of tprK within all isolates examined except the laboratory Nichols strain, which has only one tprK allele (7, 10). The variability of TprK is limited to seven discrete variable regions (V1 to V7) (7, 10). Our immunization studies in the rabbit model have shown that, when the recombinant N terminus of TprK is used as an immunogen, treponemal growth is limited and lesion development is attenuated at the sites of homologous intradermal challenge (6, 14). Epitope mapping studies revealed that, during experimental infection, T cells are directed to the conserved regions of TprK, while the antibodies are directed to the variable (V) regions (15). It has also been shown by Centurion-Lara et al. that anti-TprK antibodies are opsonic, enhancing phagocytosis of treponemes (6). Hazlett et al., however, failed to show protection after immunization with TprK, and antisera from these susceptible animals failed to opsonize T. pallidum (10). We hypothesize that the V regions of TprK and the specific antibody responses directed against them are involved in immune protection and that the absence of antibody cross-reactivity to diverse TprK V regions results, at least in part, in the absence of heterologous protection.
To test this hypothesis, we immunized three groups of rabbits with the recombinant N terminus of the Nichols strain TprK. One group of rabbits was challenged with the well-studied, homologous Nichols strain, which has one tprK sequence. This strain, however, has been propagated in rabbits for 90 years and, therefore, may not be representative of typical patient isolates. To determine whether populations of treponemes with mixed tprK sequences have an advantage in evading the immune system and establishing infection, a second group of rabbits was challenged with a typical patient isolate, Chicago, containing multiple heterologous tprK sequences. The third group of rabbits was challenged with a clonal strain recently derived from the Chicago isolate. This strain permits us to discern the effects of immunization on challenge with organisms having a single, defined, but heterologous TprK. Using strain-specific peptides, we also determined the level of cross-reactivity of antibodies directed against the V regions of TprK.
MATERIALS AND METHODS
Strains and production of immune sera.
Six outbred adult male New Zealand White rabbits (R & R Rabbitry, Stanwood, Wash.) were infected intratesticularly with 108 T. pallidum subsp. pallidum. Three rabbits were infected with the Seattle Nichols strain. Another set of three rabbits was infected with a population clonal for tprK isolated from the Chicago patient isolate. To generate the clonal Chicago strain, rabbits were infected intravenously with 108 treponemes of the Chicago isolate that were heterogeneous in their tprK genes. Biopsy specimens from the resulting disseminated skin lesions were each shown to have treponemes that were clonal for tprK, and these organisms were propagated in rabbits. To make certain the population was truly clonal, it was passed intravenously through another rabbit, and treponemes were again isolated from a single skin lesion. The clonal Chicago treponeme population has one tprK allele that differs from the Seattle Nichols strain in each of the seven V regions (Fig. 1B). After 180 days of infection, by which time rabbits are immune to homologous challenge, blood was collected from rabbits for measurement of peptide-specific reactivity. All animal studies were approved by the institutional review board at the University of Washington.
FIG. 1.
Peptide sequences. (A) Peptides made up of amino acids conserved in the Seattle Nichols and clonal Chicago strains. All conserved peptides are 20 aa long, except peptide 40, which is 54 aa long. The peptide number is shown to the left of the sequence. (B) Peptides with amino acids that differ between the two strains (variable region peptides). The peptide number is shown to the left of the sequence. Whether the sequence is a Nichols-specific (N) or clonal Chicago-specific (C) sequence is indicated. All of the Nichols peptides are 20 aa, but some of the clonal Chicago peptides have been adjusted in length to account for insertions or deletions. The number following v indicates the relevant TprK variable region. Amino acids within TprK variable regions (asterisks) and gaps introduced in the sequences to maximize alignment (dashes) are indicated.
Peptides for epitope mapping.
Overlapping synthetic peptides were designed on the basis of the Seattle Nichols and clonal Chicago strain sequences (GenBank/EMBL/DDBJ accession numbers AF194369 and AY163877, respectively). Starting after the signal sequence cleavage site at amino acid (aa) 30 (7, 10), peptides (generally 20 aa long and overlapping by 10 aa) spanning TprK were synthesized using a Rainin-PTI Symphony instrument and Sephadex desalting step to a minimum purity of 70%. Peptides were analyzed by high-performance liquid chromatography trace and mass spectrometry (Fred Hutchinson Cancer Research Center, Seattle, Wash.) and rehydrated in phosphate-buffered saline, pH 7.2. The conserved 3′ tprK region (encoding aa 425 to 478) was cloned into the pRSET expression vector, expressed in Escherichia coli, and purified as previously described (15). A total of 55 peptides were made: 24 conserved synthetic peptides and 1 conserved recombinant 54-aa peptide (Fig. 1A) and 15 synthetic Seattle Nichols-specific peptides and 15 synthetic clonal Chicago-specific peptides (Fig. 1B).
Immunization and challenge.
Recombinant protein was expressed and used to immunize rabbits as previously described (14). Briefly, the amplicon encoding aa 37 to 273 of TprK (N-terminal region) from T. pallidum Seattle Nichols strain DNA was cloned into the pRSET expression vector (Invitrogen, Carlsbad, Calif.), the sequence was verified, and the protein was expressed in E. coli and purified by nickel chromatography. The protein was dialyzed into phosphate-buffered saline (pH 7.2), confirmation of size and purity were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and concentrations were determined using a bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Twelve New Zealand White male rabbits (R & R Rabbitry) were immunized with 125 μg of the recombinant protein in the monophosphoryl lipid A-trehalose dicorynomycolate-mycobacterial cell wall skeleton emulsion adjuvant system (Sigma, St. Louis, Mo.) per immunization. Each immunization was administered subcutaneously, intradermally, intramuscularly, and intraperitoneally per the manufacturer's suggestions. Immunizations were administered every 3 weeks for a total of six immunizations. Twenty-three to twenty-six days after the last booster dose, three groups of four immunized rabbits and four unimmunized control rabbits were challenged intradermally at eight sites on their shaved backs with 105 organisms per site of T. pallidum Seattle Nichols (homologous to the immunizing peptide), clonal Chicago, or Chicago (both heterologous to the immunizing peptide). An aspirate was taken from each site of intradermal challenge 18 or 19 days after challenge and examined using dark-field microscopy for the presence of treponemes; the observer was unaware of the source of each sample. Statistical analyses were done using the chi-square test with Bonferroni's correction for analyzing multiple outcomes. Blood was collected prior to and 36 to 41 days after challenge for antibody analyses.
ELISAs
Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously with the exception that the amount of nonfat milk in the test serum diluent was increased to 10% (15). Briefly, the wells were coated with 50 μl of a solution containing 10 μg of peptides per ml, blocked with nonfat milk, washed, incubated with test serum diluted 1:20, washed, incubated with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G diluted 1:2,000, washed, and developed with para-nitrophenylphosphate substrate. In preliminary studies, the dilution of 1:20 of rabbit sera was determined to give the optimal optical density while remaining within the dynamic range such that differences in serum reactivity can be detected. Sera were preadsorbed with a crude lysate of E. coli expressing an unrelated recombinant protein to remove antibodies directed against E. coli and vector-encoded regions within recombinant peptide 40. A bicinchoninic acid protein assay (Pierce) was performed in plates that were coated with antigen and washed to demonstrate that all peptides bound to the plates (data not shown). The mean absorbance at 405 nm ± standard error of the mean of three experimental wells minus the mean of the three wells with no peptide was calculated for all conditions tested with serum from each animal. The data presented in the graphs in Fig. 2 and 3 are the means ± standard errors of the means for three animals for each condition.
FIG. 2.
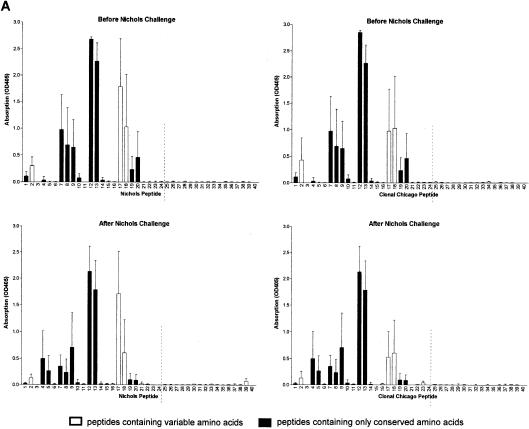
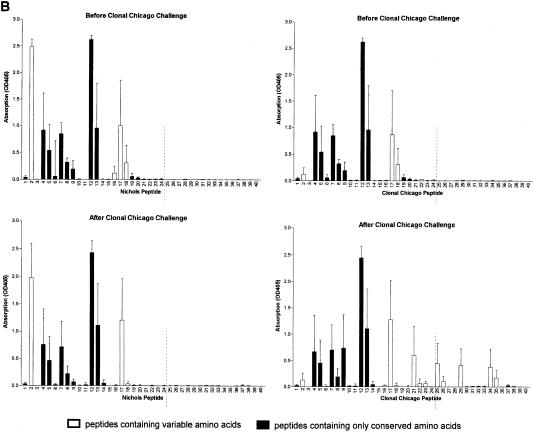
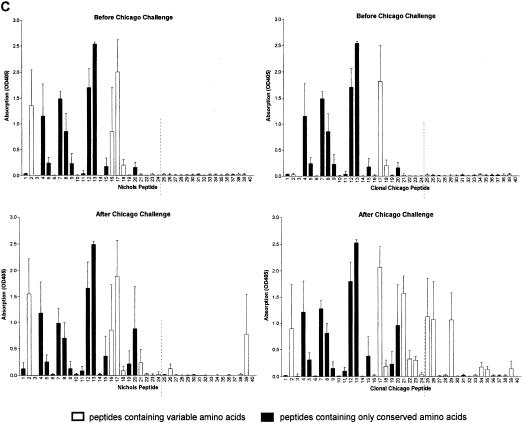
Antibody responses after challenge correlate with treponeme detection. Sera were collected from groups of three rabbits immunized with the N-terminal region of Nichols TprK before and 36 to 41 days after challenge with the Nichols strain (A), clonal Chicago strain (B), or Chicago isolate (C) and were tested in ELISAs against TprK peptides specific for either the Nichols (left) or clonal Chicago (right) strain. (A to C, top graphs) Antibodies from immunized rabbits before challenge recognize conserved and V-region peptides within the Nichols N-terminal immunogen (peptides 1 to 24 shown to the left of the broken lines). (A, bottom panels) Immunized rabbits challenged with the Nichols strain did not develop additional antibodies to peptides in the C terminus of TprK not included in the immunogen (right of the dashed lines). (B, bottom graphs) Immunized rabbits challenged with the clonal Chicago strain produced new antibodies specific for C-terminal Chicago-based peptides. Immunized rabbits challenged with the Chicago isolate produced new antibodies against multiple C-terminal Chicago peptides and only two Nichols-based peptides. (C, bottom graphs). OD405, optical density at 405 nm.
FIG. 3.
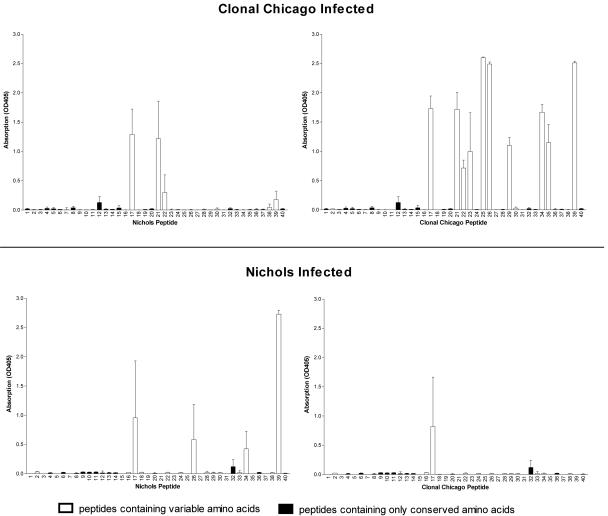
Limited cross-reactivity of antibodies to variable B-cell epitopes between heterologous strains. Sera collected from groups of three rabbits infected for 180 days with the clonal Chicago or Nichols strain were tested against a panel of peptides spanning TprK, including conserved regions and peptides specific for either the clonal Chicago (right) or Nichols (left) strain. There is limited cross-reactivity of antibodies that recognize clonal Chicago-specific sequences and Nichols-specific sequences. OD405, optical density at 405 nm.
RESULTS
tprK V-region differences in the challenge inoculum correlate with decreased effects of immunization with the N-terminal region of TprK.
We have repeatedly shown that TprK, and specifically the N-terminal region of TprK (aa 37 to 273), when used as an immunogen, attenuates lesion development against homologous intradermal challenge in the rabbit model (6, 14). To determine whether the V regions within TprK play a role in that protection, we immunized rabbits with the recombinant N-terminal region of Nichols TprK previously shown to confer maximal lesion attenuation (14) and challenged intradermally with either the homologous Nichols strain, the heterologous clonal Chicago strain (whose single tprK allele differs from the Nichols strain in all seven V regions), or the heterologous Chicago isolate (with multiple tprK alleles that differ from the Nichols strain). All groups of immunized rabbits, regardless of challenge inoculum, had significantly fewer challenge sites with treponemes by dark-field microscopy than the unimmunized controls challenged with the same strains (Table 1). The challenge sites of all immunized rabbits developed erythema by day 2 or 3 after challenge, and this erythema resolved early, whereas the challenge sites of unimmunized animals generally did not develop erythema until days 7 to 10 and lesions progressed to ulceration. Among the immunized rabbits, those challenged with the homologous Nichols strain had fewer lesions with treponemes than those challenged with either the heterologous clonal Chicago strain or Chicago isolate (P < 0.001) (Table 1).
TABLE 1.
Presence of treponemes after intradermal challenge
| Challenge strain or isolate | Treponeme positive/totala (% positive)
|
|
|---|---|---|
| Immunized | Control | |
| Nichols | 5/32 (16)b,d,e | 28/32 (88) |
| Clonal Chicago | 11/32 (34)b,c | 24/32 (75) |
| Chicago | 13/32 (41)b,c | 26/32 (81) |
Values are the number of sites with treponemes/total number of sites. The presence of treponemes was detected by dark-field microscopy in aspirates taken from inoculation sites, and 32 inoculation sites were sampled for each group.
Significantly different (P < 0.001) from the value for unimmunized controls by the chi-square test with Bonferroni's correction.
Significantly different (P < 0.001) from the value for immunized rabbits challenged with the Nichols strain by the chi-square test with Bonferroni's correction.
Significantly different (P < 0.001) from the value for immunized rabbits challenged with the clonal Chicago strain by the chi-square test with Bonferroni's correction.
Significantly different (P < 0.001) from the value for immunized rabbits challenged with the Chicago isolate by the chi-square test with Bonferroni's correction.
Expansion of antibody specificities in immunized rabbits after challenge correlates with increased tprK diversity.
Immunization induced the development of antibodies that recognized a large number of peptides within the N-terminal immunogen, including both conserved and variable domains of TprK (Fig. 2, top graphs). After challenge with the Nichols strain, rabbits failed to develop responses to peptides of the C-terminal portion of TprK that would have been present in the challenge inoculum but not in the immunizing peptides (Fig. 2A, bottom graphs); these results further suggest that homologous challenge infection was well controlled. In contrast, immunized rabbits challenged with the clonal Chicago strain developed moderate antibody responses recognizing three additional V regions (Fig. 2B, bottom graphs), and those challenged with the heterogeneous Chicago isolate developed responses to all four V regions within the C terminus (Fig. 2C, bottom graphs). Thus, the number of new epitopes recognized and the magnitude of the antibody response correlated with increased level of heterogeneity of tprK in the challenge inoculum and were inversely correlated with attenuation of challenge infection. Sera collected prior to immunization were uniformly nonreactive to TprK peptides, while the intradermally challenged control rabbits in all groups developed antibody responses to V-region epitopes (data not shown).
Antibodies directed to TprK V regions show limited cross-reactivity between strains.
It was previously shown that antibodies are directed to the V regions of TprK after acute infection with the Nichols strain in the rabbit model (15). To determine whether antibody responses were specific for particular V-region sequences or whether sera would cross-react with multiple V-region sequences, we tested sera from rabbits infected with either the clonal Chicago or Nichols strain in ELISAs against clonal Chicago- and Nichols-specific TprK peptides. Sera from rabbits infected with either strain reacted specifically to multiple homologous V-region peptides and showed limited cross-reactivity only to a subset of heterologous V regions (Fig. 3). Sera from uninfected rabbits did not react with the TprK peptides (data not shown).
DISCUSSION
After it was discovered that TprK has discrete V regions (7), we began to explore the possibility that the diversity of TprK is involved in immune evasion. We previously demonstrated that rabbits immunized with a fragment consisting of the N-terminal region of TprK have decreased treponemal burden at the sites of intradermal challenge (14). For the studies presented in this report, we sought to examine whether the V regions of TprK are involved in this protection and, conversely, whether diversity in the V-region sequences is associated with the lack of heterologous protection. We found that differences in the V regions of tprK in the challenge inoculum correlate with reduced control of treponemal growth.
We previously demonstrated that, during experimental infection, T-cell epitopes are located in the conserved regions of TprK, while B-cell epitopes are in the V regions (15). In this report, we have confirmed that, after acute infection, antibodies are primarily directed to V-region peptides, whether rabbits are infected intratesticularly with a large inoculum (Fig. 3) or intradermally with a small inoculum (control rabbits for immunization study [data not shown]). In addition, we found there was limited antibody cross-reactivity to V-region peptides from the Nichols strain and the clonal Chicago strain (Fig. 3). An analogous situation exists with the Chlamydia trachomatis major outer membrane protein (MOMP). MOMP has four discrete variable regions that are surface exposed and contain protective B-cell epitopes of limited cross-reactivity (1, 16, 17), whereas the conserved regions contain T-cell epitopes (18). Furthermore, the variability of MOMP has been linked to the lack of heterologous C. trachomatis protection in humans (5).
In our study, there was a statistically significant decrease in treponemes in all immunized groups of rabbits compared to their respective controls (Table 1). T-cell responses to conserved epitopes may account for this decrease in treponemes. These conserved T-cell epitopes may be useful components in a multisubunit vaccine. Strain-specific antibody responses to the V regions, however, may account for the higher level of protection seen after homologous challenge. The numbers of lesions with treponemes in unimmunized control rabbits were not statistically significantly different. These data suggest that the statistically different results among the immunized rabbits are not due to differences in the virulence of the strains.
After the immunized rabbits were challenged, development of new antibody specificities correlated with an increased likelihood of treponemes being found at the sites of intradermal challenge (Fig. 2). Immunized rabbits challenged with the Nichols strain had the fewest number of lesions by dark-field microscopy and did not develop antibodies to any new epitopes not included in the N-terminal immunogen. The data strongly suggest that the Nichols challenge inoculum was quickly and effectively cleared by immunization-induced immune mechanisms. Although it is possible that the Nichols strain was inherently more susceptible to immunization-induced clearance mechanisms, the correlation between TprK antibody recognition and treponemal clearance suggests that the major factor was immune recognition of Nichols TprK but not Chicago TprK after immunization. In contrast to the immunized rabbits challenged with the Nichols isolate, immunized rabbits challenged with the Chicago isolate developed a robust antibody response to many new TprK peptides, similar to that seen in control animals (data not shown). These results suggest that differences in the TprK V regions of the Chicago challenge inoculum compared with the Nichols TprK immunogen allow more treponemes to escape the existing immune surveillance, proliferate, and then induce development of additional new antibody specificities.
In examining the antibody response to small peptides, we recognize that we may be losing information about antibodies to conformational epitopes. The conclusion, however, about the association of protection with V-region-specific antipeptide antibodies would not change with information about conformation-specific antibodies.
Antibodies from rabbits challenged with the heterologous Chicago isolate were able to recognize both clonal Chicago-specific peptides and the Nichols V7 peptide 39, while rabbits challenged with the clonal Chicago isolate recognized only clonal Chicago-specific peptides (Fig. 2). Sequencing data demonstrate that the Chicago isolate is a mixed population of treponemes that contains different tprK alleles (A. Centurion-Lara, C. Godornes, B. Molini, and S. A. Lukehart, unpublished data). It is not surprising that treponemes within the heterogeneous Chicago population elicited antibodies reactive to peptides from the derived clonal Chicago strain. Rabbits challenged with the Chicago isolate also developed antibodies that recognized Nichols-specific V7 peptide 39. In rabbits infected with the Nichols isolate, peptide 39 is the peptide that most often elicits strong antibody responses (15). It may require only a relatively small number of treponemes in the Chicago isolate with the same or similar V7 as the Nichols strain to elicit an antibody response to such an immunodominant epitope.
Antibodies from rabbits infected with either the Nichols or clonal Chicago strain show very limited cross-reactivity to the corresponding heterologous V regions. The most cross-reactivity was seen with V2 peptide 17, which differs by only 3 aa in these strains, the smallest difference in any of the seven V regions. There was no antibody cross-reactivity to more-divergent V regions.
A major mechanism for clearance of T. pallidum from tissue is a T-cell-mediated response that activates macrophages to phagocytose antibody-opsonized treponemes (2, 9, 12, 20). The conserved T-cell epitopes within TprK may stimulate the immune system to produce gamma interferon for activation of macrophages. T. pallidum are not efficiently phagocytosed and killed by macrophages without antibodies (3, 11). It has been reported that TprK is a target of opsonic antibodies (6). Treponemes expressing TprKs differing from the sequence of the immunogen or of treponemes from a previous infection may be able to resist opsonization by antibodies directed against the V regions of a previously seen TprK. Thus, phagocytosis would also be avoided, and new infection would ensue. It is also possible that the antibodies directed against the TprK V regions could have other functions, such as neutralizing activity, blocking attachment to host cells, or interfering with another yet undefined process of infection.
Antigenic diversity as a means of escaping the host immune response is used by a variety of pathogens, including protozoa, viruses, fungi, and bacteria, including other spirochetes (8). It has been speculated for decades that the limited protection against heterologous T. pallidum isolates in rabbits and humans is due to antigenic diversity among isolates, but no candidate antigens had been proposed. The data in our study are the first immunological evidence implicating a specific T. pallidum protein in the degree of protection against heterologous strains.
Acknowledgments
We thank Charmie Godornes and Barbara Molini for excellent work developing the clonal Chicago strains.
This work was supported in part by U.S. Public Health Service grants AI-34616 and AI-42143 (S. A. Lukehart) and AI-43456 (W. C. Van Voorhis). C. A. Morgan was supported by an institutional training grant AI-07509 from the National Institutes of Health and by the Magnuson Scholars Award from the University of Washington.
Editor: D. L. Burns
REFERENCES
- 1.Baehr, W., Y. X. Zhang, T. Joseph, H. Su, F. E. Nano, K. D. Everett, and H. D. Caldwell. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. USA 85:4000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker-Zander, S., and S. Sell. 1980. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am. J. Pathol. 101:387-413. [PMC free article] [PubMed] [Google Scholar]
- 3.Baker-Zander, S. A., and S. A. Lukehart. 1992. Macrophage-mediated killing of opsonized Treponema pallidum. J. Infect. Dis. 165:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, N. H., and J. N. Miller. 1976. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J. Immunol. 117:191-196. [PubMed] [Google Scholar]
- 5.Brunham, R. C., J. Kimani, J. Bwayo, G. Maitha, I. Maclean, C. Yang, C. Shen, S. Roman, N. J. Nagelkerke, M. Cheang, and F. A. Plummer. 1996. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J. Infect. Dis. 173:950-956. [DOI] [PubMed] [Google Scholar]
- 6.Centurion-Lara, A., C. Castro, L. Barrett, C. Cameron, M. Mostowfi, W. C. Van Voorhis, and S. A. Lukehart. 1999. Treponema pallidum major sheath protein homologue TprK is a target of opsonic antibody and the protective immune response. J. Exp. Med. 189:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centurion-Lara, A., C. Godornes, C. Castro, W. C. Van Voorhis, and S. A. Lukehart. 2000. The tprK gene is heterogeneous among Treponema pallidum strains and has multiple alleles. Infect. Immun. 68:824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deitsch, K. W., E. R. Moxon, and T. E. Wellems. 1997. Shared themes of antigenic variation and virulence in bacterial, protozoal, and fungal infections. Microbiol. Mol. Biol. Rev. 61:281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelkens, H. J., F. J. ten Kate, J. Judanarso, V. D. Vuzevski, J. B. van Lier, J. C. Godschalk, J. J. van der Sluis, and E. Stolz. 1993. The localisation of treponemes and characterisation of the inflammatory infiltrate in skin biopsies from patients with primary or secondary syphilis, or early infectious yaws. Genitourin. Med. 69:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazlett, K. R., T. J. Sellati, T. T. Nguyen, D. L. Cox, M. L. Clawson, M. J. Caimano, and J. D. Radolf. 2001. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 193:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukehart, S. A., and J. N. Miller. 1978. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J. Immunol. 121:2014-2024. [PubMed] [Google Scholar]
- 12.Lukehart, S. A., S. A. Baker-Zander, R. M. Lloyd, and S. Sell. 1980. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J. Immunol. 124:461-467. [PubMed] [Google Scholar]
- 13.Magnuson, H. J., E. W. Thomas, S. Olansky, B. I. Kaplan, L. De Mello, and J. C. Cutler. 1956. Inoculation syphilis in human volunteers. Medicine 35:33-82. [DOI] [PubMed] [Google Scholar]
- 14.Morgan, C. A., S. A. Lukehart, and W. C. Van Voorhis. 2002. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect. Immun. 70:6811-6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan, C. A., B. J. Molini, S. A. Lukehart, and W. C. Van Voorhis. 2002. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J. Immunol. 169:952-957. [DOI] [PubMed] [Google Scholar]
- 16.Stephens, R. S., R. Sanchez-Pescador, E. A. Wagar, C. Inouye, and M. S. Urdea. 1987. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 169:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens, R. S., E. A. Wagar, and G. K. Schoolnik. 1988. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J. Exp. Med. 167:817-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su, H., R. P. Morrison, N. G. Watkins, and H. D. Caldwell. 1990. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J. Exp. Med. 172:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner, T. B., and D. H. Hollander. 1957. Biology of treponematoses. World Health Organization, Geneva, Switzerland.
- 20.Van Voorhis, W. C., L. K. Barrett, D. M. Koelle, J. M. Nasio, F. A. Plummer, and S. A. Lukehart. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491-495. [DOI] [PubMed] [Google Scholar]
- 21.Wicher, V., K. Wicher, A. Jakubowski, and S. M. Nakeeb. 1987. Adoptive transfer of immunity to Treponema pallidum Nichols infection in inbred strain 2 and C4D guinea pigs. Infect. Immun. 55:2502-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wicher, K., and V. Wicher. 1989. Experimental syphilis in guinea pig. Crit. Rev. Microbiol. 16:181-234. [DOI] [PubMed] [Google Scholar]