Abstract
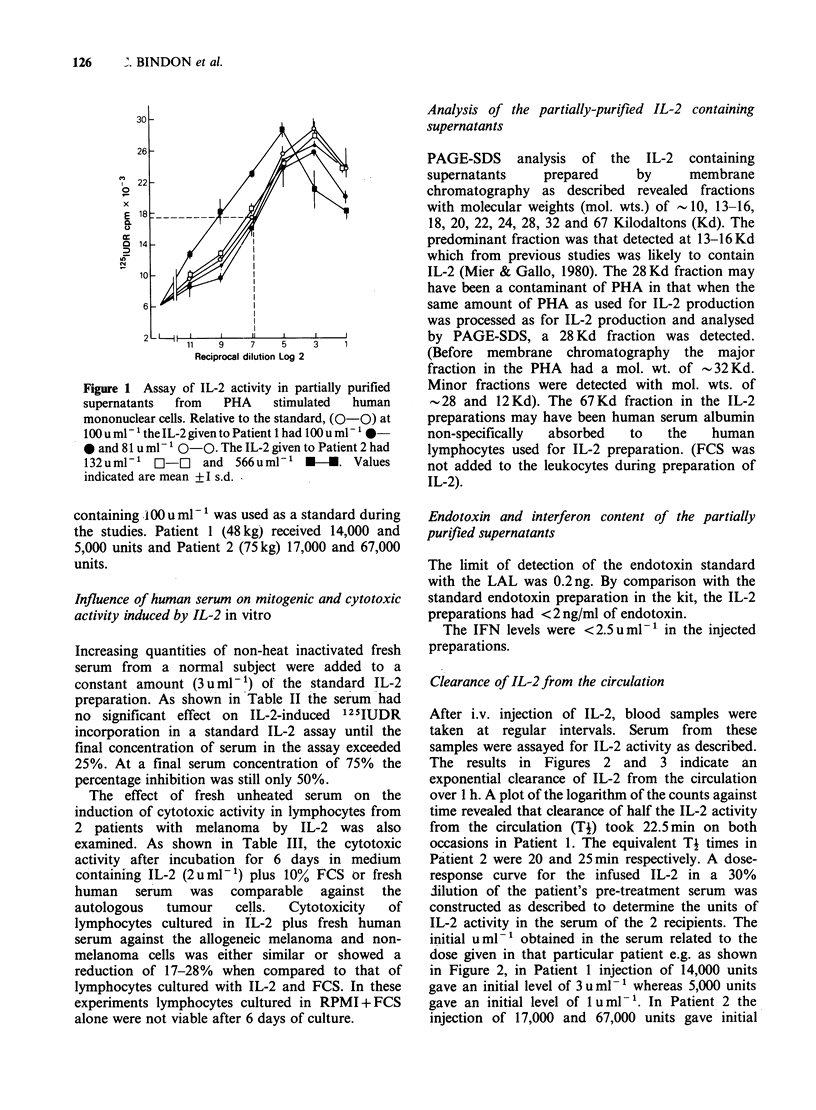
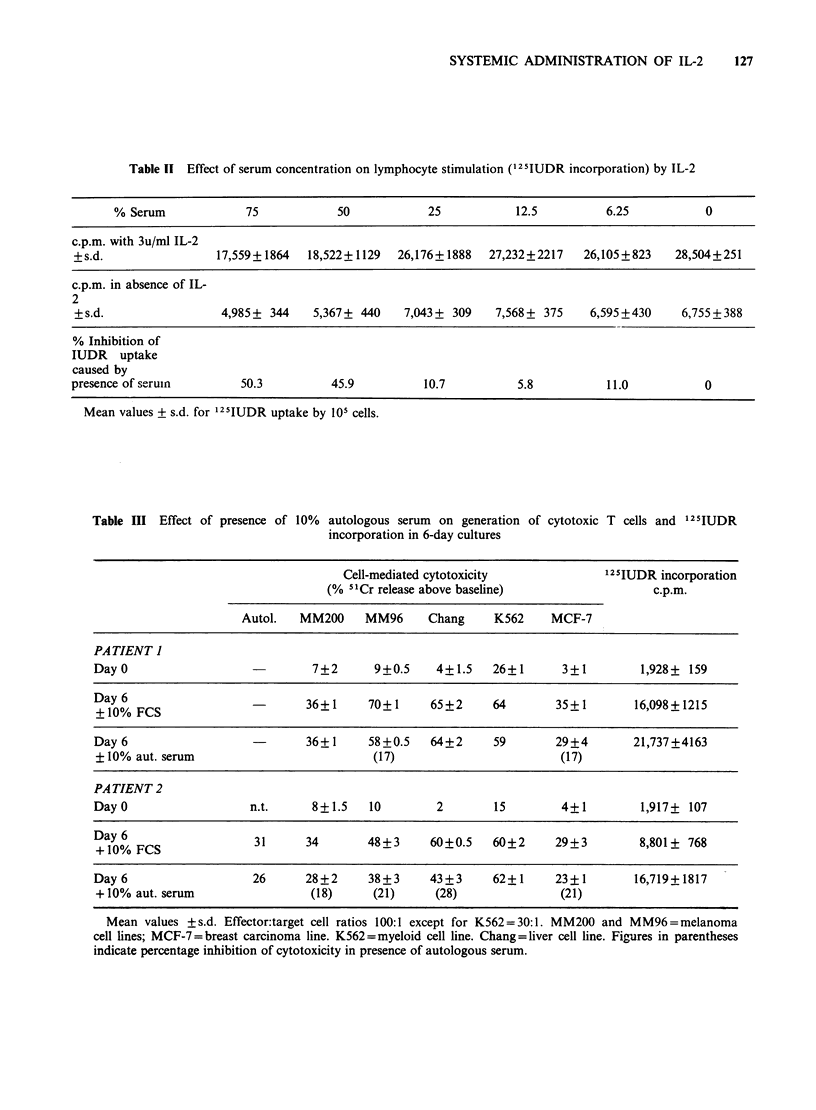
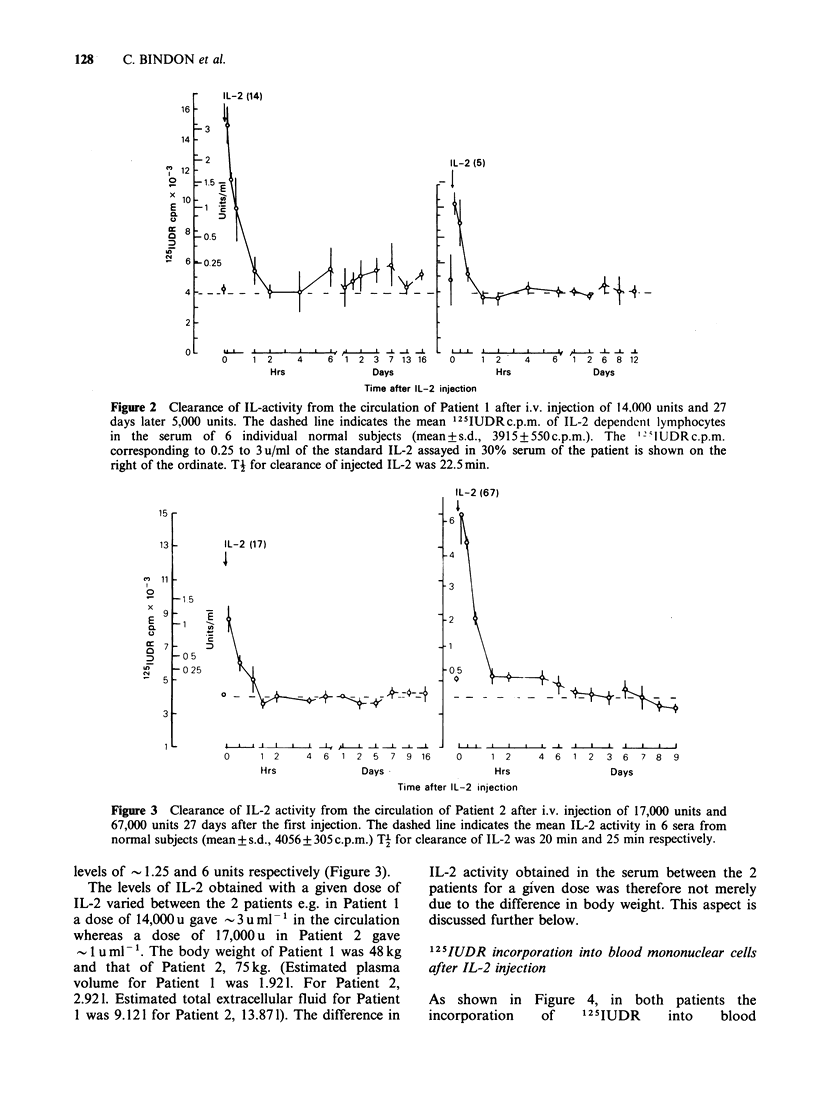
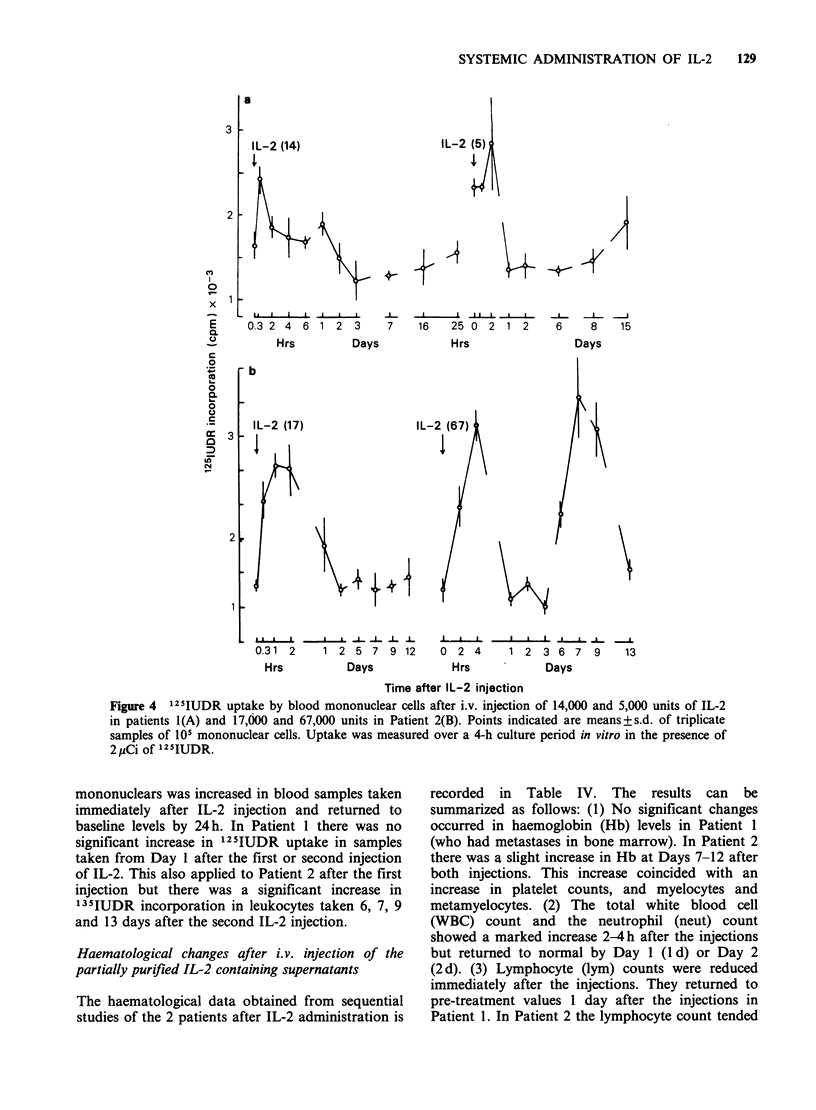
The present study was designed to examine the feasibility of in vivo administration of interleukin 2 (IL-2) to induce cytotoxic cell activity against tumours in human subjects. IL-2 was prepared from blood leukocytes stimulated with phytohaemagglutinin (PHA) and partially purified by membrane chromatography to exclude PHA. Administration of different amounts of IL-2 in vivo to 2 patients with melanoma revealed that the initial level of IL-2 in the circulation was related to the dose given and had a half-life of approximately 22.5 minutes. The initial and subsequent levels of IL-2 were lower than that expected to occur from equilibration in plasma and extracellular fluid. This was not apparently due to inactivation by serum factors because fresh human serum had little effect in vitro on the induction of mitogenic or cytotoxic activity by IL-2. Spontaneous division of lymphocytes was increased following IL-2 administration and it is suggested that clearance of IL-2 in vivo may reflect, in part, absorption by activated lymphocytes in the circulation. Side effects noted shortly after administration of the partially-purified IL-2 preparations included transient pyrexia, hypoglycaemia, increased cortisol levels, lymphocytopenia and signs of mild intravascular coagulation. No long-term effects were noted. These initial results suggest that systemic injection of purified preparations of II-2 may be a feasible approach to induce cytotoxic T cells in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besedovsky H. O., del Rey A., Sorkin E. Lymphokine-containing supernatants from con A-stimulated cells increase corticosterone blood levels. J Immunol. 1981 Jan;126(1):385–387. [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Gillis S., Watson J. Interleukin-2 dependent culture of cytolytic T cell lines. Immunol Rev. 1981;54:81–109. doi: 10.1111/j.1600-065x.1981.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Hersey P., Bindon C., Edwards A., Murray E., Phillips G., McCarthy W. H. Induction of cytotoxic activity in human lymphocytes against autologous and allogeneic melanoma cells in vitro by culture with interleukin 2. Int J Cancer. 1981 Dec;28(6):695–703. doi: 10.1002/ijc.2910280607. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Lewis R., Kemp A., McInnes J. Deficient natural killer cell activity in a patient with Fanconi's anaemia and squamous cell carcinoma. Association with defect in interferon release. Clin Exp Immunol. 1982 Apr;48(1):205–212. [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. J., Andrus L., Prowse S. J. Role of lymphokine and antigen in the control of specific T cell responses. Immunol Rev. 1980;51:279–314. doi: 10.1111/j.1600-065x.1980.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Line B. R., Mathisen D. J., Rosenberg S. A. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors. J Immunol. 1980 Oct;125(4):1487–1493. [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G. B., Carlson G., Paetkau V. Generation of cytotoxic lymphocytes to syngeneic tumors by using co-stimulator (Interleukin 2): in vivo activity. J Immunol. 1980 Nov;125(5):1904–1909. [PubMed] [Google Scholar]
- Mills G. B., Paetkau V. Generation of cytotoxic lymphocytes to syngeneic tumor by using co-stimulator (Interleukin 2). J Immunol. 1980 Nov;125(5):1897–1903. [PubMed] [Google Scholar]
- Moore R. N., Goodrum K. J., Couch R., Jr, Berry L. J. Factors affecting macrophage function: glucocorticoid antagonizing factor. J Reticuloendothel Soc. 1978 Apr;23(4):321–332. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Bonnard G. D. Specific cytotoxicity against autologous tumour and proliferative responses of human lymphocytes grown in interleukin 2. Int J Cancer. 1982 Jan 15;29(1):33–39. doi: 10.1002/ijc.2910290107. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Moore M. Cultured human T-cell lines kill autologous solid tumours. Immunol Lett. 1981 Oct;3(4):237–241. doi: 10.1016/0165-2478(81)90081-x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Röllinghoff M., Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980 Mar 20;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Woolnough J. A., Denaro C. P., Lafferty K. J. Cytotoxic T cell responses to a syngeneic tumour: conditions for primary activation in vitro and biological activity in vivo. Adv Exp Med Biol. 1979;114:757–761. doi: 10.1007/978-1-4615-9101-6_124. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Woolnough J. A., Lafferty K. J. Cytotoxic T cell responses to a syngeneic tumour: conditions for primary activation in vitro. Aust J Exp Biol Med Sci. 1978 Apr;56(2):247–251. doi: 10.1038/icb.1978.26. [DOI] [PubMed] [Google Scholar]
- Watson J., Mochizuki D. Interleukin 2: a class of T cell growth factors. Immunol Rev. 1980;51:257–278. doi: 10.1111/j.1600-065x.1980.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Bach F. H. Continuous culture of T cells cytotoxic for autologous human leukaemia cells. Nature. 1979 Aug 23;280(5724):685–688. doi: 10.1038/280685a0. [DOI] [PubMed] [Google Scholar]


