Abstract
Susceptibility testing was performed at seven Canadian microbiology laboratories and the Helicobacter Reference Laboratory, Halifax, Nova Scotia, Canada, to assess susceptibility testing proficiency and the reproducibility of the results for clarithromycin and metronidazole and to compare the Epsilometer test (E test) method to the agar dilution reference method. Control strain Helicobacter pylori ATCC 43504 (American Type Culture Collection) and 13 clinical isolates (plus duplicates of four of these strains including ATCC 43504) were tested blindly. The National Committee for Clinical Laboratory Standards (NCCLS) guidelines for agar dilution testing were followed, and the same suspension of organisms was used for agar dilution and E test. Antimicrobials and E test strips were provided to the investigators. Methods were provided on a website (www.Helicobactercanada.org). Each center reported MICs within the stated range for strain ATCC 43504. Compared to the average MICs, interlaboratory agreements within 2 log2 dilutions were 90% (range, 69 to 100%) for clarithromycin by agar dilution, with seven very major errors [VMEs], and 85% (range, 65 to 100%) by E test, with three VMEs. Interlaboratory agreements within 2 log2 dilutions were 83% (range, 50 to 100%) for metronidazole by agar dilution, with six VMEs and eight major errors (MEs), and 75% (range, 50 to 94%) by E test, with four VMEs and four MEs. At lower and higher concentrations of antibiotic, E test MICs were slightly different from agar dilution MICs, but these differences did not result in errors. When a standardized protocol based on NCCLS guidelines was used, most participants in this study correctly identified clarithromycin- and metronidazole-susceptible and -resistant strains of H. pylori 93% of the time by either the agar dilution or E test method, and the numbers of errors were relatively equivalent by both methods.
Helicobacter pylori causes gastritis and ulcer disease and is considered a risk factor for the development of mucosa-associated lymphoma and gastric cancer (10, 23, 25, 29, 31, 47). Most consensus guidelines now recommend eradication of H. pylori in all patients known to be infected. The most commonly used treatments are triple therapies consisting of a proton pump inhibitor with clarithromycin and metronidazole or amoxicillin or quadruple therapy with a proton pump inhibitor, a bismuth compound, tetracycline, and metronidazole (3, 8, 21, 39). The primary and secondary resistance rates reported worldwide vary from 1 to 58% for clarithromycin and from 5 to 76% for metronidazole (1, 5, 15, 17, 26, 28, 32, 43). Resistance to one or both of these antibiotics significantly reduces treatment success (2, 11, 19, 22, 35, 37, 42, 44). The clinical importance of resistance makes it necessary for antibiotic treatment decisions to be based on valid and reproducible in vitro susceptibility testing results. Reported discrepant susceptibility test results are due to variations in the methods and conditions used for susceptibility testing (16, 18, 20, 24, 33, 40, 41, 49).
The National Committee for Clinical Laboratory Standards (NCCLS) has recently published guidelines for in vitro susceptibility testing of H. pylori using an agar dilution method (30). Agar dilution tests are time-consuming and labor-intensive, and a large number of strains should be tested simultaneously since the media containing the antibiotic dilutions must be prepared immediately before use. The procedure is efficient for the testing of large numbers of strains, such as in susceptibility surveys, but it is not readily adaptable for the testing of small numbers of strains on an ongoing basis. The Epsilometer test (E test) method (AB Biodisk, Solna, Sweden) has been approved for use for the susceptibility testing of many organisms, but not H. pylori. However, many studies have been carried out by this method with H. pylori (6, 7, 12, 14, 27, 34, 36, 38). The proprietary antibiotic-impregnated E test strips are expensive, but less labor is required relative to the amount of labor required for agar dilution, and the MIC is read directly from the confluence of growth with the strip. Since single tests are easily carried out, E test might be a preferable method for the testing of small numbers of strains.
There are limited data on H. pylori resistance in Canada (4, 5, 9). It was decided that before a survey for the rates of H. pylori resistance to clarithromycin and metronidazole in Canada was conducted, seven Canadian microbiology laboratories would assess their susceptibility testing proficiencies with 13 strains of H. pylori and reference strain ATCC 43504 and would compare the E test method with the agar dilution reference method.
MATERIALS AND METHODS
Media and antibiotics.
Methods based on the NCCLS guidelines were distributed by using a specially designed website (www.Helicobactercanada.org) established for that purpose. Strict adherence to the methods was mandated. Antibiotic powders and E test strips from the same lots were sent to the participants. The NCCLS (30) guidelines for preparation of antibiotics and plates were followed. Brucella broth with 5% fetal calf serum (BBFC; Oxoid Canada, Nepean, Ontario, Canada) with 20% glycerol was used to freeze H. pylori strains for storage at −70°C. Mueller-Hinton agar (Oxoid) with 5% sheep blood aged more than 2 weeks (MHB) was used to grow the frozen strains and for susceptibility testing. All MHB agar dilution and E test plates used for testing were prepared with the same lots of medium and blood. All antibiotic powders and E test strips sent to the participants were from the same manufactured lots. The metronidazole powder (Sigma Chemical Company, St. Louis, Mo.) used to make a 51,200-μg/ml stock solution was added to sterile distilled water and dissolved by stirring and warming to less than 50°C (one laboratory used 150 μl of dilute (10%) acetic acid to dissolve the metronidazole). The clarithromycin powder (Abbott Laboratories, Abbott Park, Ill.) used to make a 51,200-μg/ml stock solution was added to a small amount of 100% methanol, sonicated, and brought to a final volume in 0.1 M phosphate buffer. On the day of use, the antibiotics were serially diluted in sterile water to give final concentrations from 256 to 0.016 μg/ml and were added to the MHB plates at less than 50°C. MHB plates for E tests were prepared at the same time. All plates were warmed to 35°C prior to use. Sterile saline was used to suspend the organisms for testing (one laboratory used brain heart infusion broth [Difco Laboratories] with 5% yeast extract and 5% equine serum).
H. pylori strains.
H. pylori ATCC 43504 was the reference strain used for quality control and was purchased from the American Type Culture Collection (ATCC). For H. pylori ATCC 43504, the clarithromycin MIC range is 0.016 to 0.125 μg/ml and the metronidazole MIC range is 64 to 256 μg/ml. Two cultures of the reference strain, one identified and one blinded, were sent to each site.
The Helicobacter Reference Laboratory maintains H. pylori isolates from consenting adult patients presenting at the Queen Elizabeth II Health Sciences Center; the isolates have been maintained frozen at −70°C. Thirteen H. pylori strains that were recovered from selected patients with gastroduodenal symptoms and that had a wide range of antibiotic susceptibilities when tested in the Helicobacter Reference Laboratory were used for this study. Four strains were sensitive to clarithromycin and metronidazole, two were resistant to both drugs, two were sensitive to clarithromycin and resistant to metronidazole, and five were resistant to clarithromycin and sensitive to metronidazole. Two strains with mixed resistance had smaller numbers of resistant than sensitive colonies. The average MIC for each H. pylori strain was the average of all agar dilution values that were less than 6 log2 dilutions different from the median MIC (in micrograms per milliliter) for all participants, and all determinations were compared to that MIC.
Strains were grown on MHB at 35°C under microaerobic conditions by using an anaerobic incubator or anaerobic jars evacuated to 25 lb/in2 and filled with 5% O2, 10% CO2, and 85% N2. Multiple aliquots of each strain were frozen in BBFC-glycerol at −70°C. Cultures of the 13 test strains and reference strain ATCC 43504 and blinded duplicates of 4 strains including ATCC 43504 were labeled individually for each site. The strains used for proficiency testing were couriered overnight on dry ice to the seven participating sites. The strains were tested three times in the Helicobacter Reference Laboratory of the Queen Elizabeth II Health Sciences Center.
Strains were considered resistant to clarithromycin if the MIC was equal to or greater than 1 μg/ml and resistant to metronidazole if the MIC was greater than 8 μg/ml (15, 27, 29, 30).
Agar dilution.
Strains were grown from frozen cultures on MHB without antibiotics and subcultured a minimum of two times but not more than three times. Each H. pylori strain was suspended at a density equivalent to a McFarland no. 2 standard in sterile saline. A 0.1-ml aliquot of the suspension was serially diluted and streaked onto antibiotic-free plates to assess the number of CFU per milliliter.
A Steers replicator was used to deliver approximately 5 μl of each McFarland no. 2 standard suspension onto MHB plates with antibiotics. An antibiotic-free plate was inoculated at the beginning and end of each series of plates to confirm the viability of the inoculum and to observe the growth of any contaminants. The plates were incubated under microaerobic conditions at 35°C for 72 h. The NCCLS agar dilution protocol defines the breakpoint MIC as the lowest concentration of antibiotic showing no growth, a haze, one discrete colony, or multiple tiny colonies; and where there is persistent slight growth, the MIC is read as the concentration at which a marked change in the appearance relative to that of the control plate occurs. The MIC was recorded at 72 h.
E tests.
MHB plates were inoculated with the same H. pylori suspensions used for the agar dilution method by using a sterile swab to completely cover the surface. The plates were allowed to dry very briefly before metronidazole or clarithromycin E test strips were carefully added, taking care not to move the strip once it had contacted the surface. The plates were incubated as described above. The E test MIC was interpreted as the point at which the growth intersected the strip. Where there was a haze of growth that could not be distinguished from the inoculum, the haze was discounted. For comparison of the E test MICs with the twofold agar dilution MICs determined by the NCCLS method, when the growth on an E test plate ended at an intermediate MIC, the next higher value was recorded. The MIC was recorded at 72 h.
Analysis of results.
Coded agar dilution and E test results from each center were entered into a database. The median MIC for all agar dilution results for each strain was calculated, and values more than 6 log2 dilutions different were excluded from determination of the final MIC for each strain. Analysis was performed by using InStat statistical software (GraphPad Software, San Diego, Calif.) to derive Spearman nonparametric correlations between sites and between agar dilution and E test results. All results were included in the error rate determination (a very major error [VME] was resistant by the reference method and susceptible by the test; a major error [ME] was susceptible by the reference method and resistant by the test) (6, 34). Laboratory-specific results for the agar dilution and E test methods were assessed relative to the average MIC for each strain, and values within 2 log2 dilutions were considered within the acceptable range. E test results were compared with agar dilution results by laboratory and by strain.
RESULTS
At the Helicobacter Reference Laboratory of the Queen Elizabeth II Health Sciences Center, colony counts performed with H. pylori strains at a density of a McFarland no. 2 standard yielded approximately 108 CFU/ml. Agar dilution plates were inoculated with 5 μl, or approximately 5 × 105 CFU, and plates with E test strips were inoculated with 100 μl, or approximately 1 × 107 CFU. At the Helicobacter Reference Laboratory, a better correlation between the agar dilution and E test methods was achieved when growth on the E test plate was observed by using a stereoscopic microscope with reflected light.
The fastidious nature of H. pylori required that careful attention be paid to the length of exposure to ambient air, the medium used, culture conditions, and assessment of growth of the tiny translucent colonies. Participants reported difficulty carrying out agar dilution quickly enough to limit exposure to ambient air and temperature. Most participants found it difficult to get clarithromycin into suspension. Agar dilution plates took longer to read, and several sites considered the interpretation of results to be more difficult by the agar dilution method than by E test, in which the results are read at the junction of bacterial growth and the antibiotic strip directly from a single plate. Contamination was only sporadically encountered; one site, however, was unable to report 40% of the expected results because of contamination.
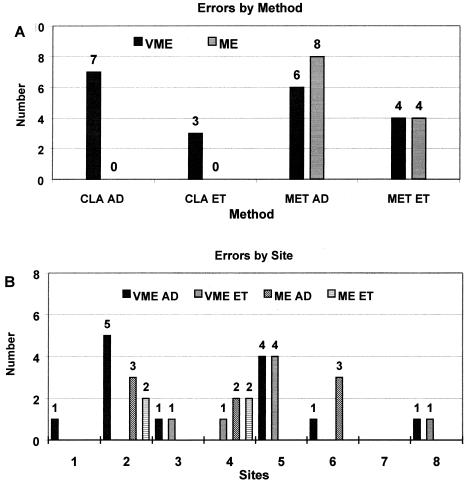
All sites achieved MICs within the accepted range for strain ATCC 43504. Two sites performed the tests in triplicate, and two repeated some or all of the tests. At these sites, proficiencies on the basis of a single set of values or the average of their results were not found to be substantially different. For all tests with the 13 strains and the blinded 4 duplicate isolates there were 757 results, of which 49 were outliers with values ≥6 log2 dilutions different from the average value. When the values for each site were averaged, there were 475 results, of which 32 (7%) were outliers. Error rate determination for each site was based on the average of the results from that site. The outliers were excluded from determination of the final MIC for each strain but were included in the error rate determination. The outliers accounted for all of the VMEs and the MEs. Relative to the average MIC and including all results for clarithromycin, there were 7 of 124 (6%) VMEs by agar dilution and 3 of 112 (3%) VMEs by E test. For metronidazole there were 6 of 125 (5%) VMEs and 8 of 125 (6%) MEs by agar dilution and 4 of 114 (3%) VMEs and 4 of 114 (4%) MEs by E test (Fig. 1A). Four of the sites correctly reported two MICs for two cultures with both resistant and susceptible strains. Failure by other sites to detect the resistant colonies accounted for 11 of 32 (34%) errors (7 VMEs by agar dilution and 4 VMEs by E test). Two sites accounted for 18 of 32 (56%) of the VMEs and MEs, with other sites having zero to five errors (Fig. 1B). As many as 9 of 32 (28%) VMEs from those two sites could have resulted from failure of organism growth, being interpreted as an MIC <0.016 μg/ml. Overall, there were 13 of 249 (5%) VMEs and 7 of 249 (3%) MEs by agar dilution. There were 8 of 226 (4%) VMEs and 4 of 226 (2%) MEs by E test. Apart from the errors, susceptible and resistant strains were identified equivalently by agar dilution and E test by all sites. In no instance did a value within 2 log2 dilutions of the average MIC cause an error.
FIG. 1.
Errors for clarithromycin (CLA) and metronidazole (MET) MICs by agar dilution (AD) and E test (ET) relative to the average MIC for each H. pylori strain by method (A) and site (B). VMEs are resistance classified as sensitivity by the test, and MEs are sensitivity classified as resistance by the test. There were 32 errors in total.
Agar dilution and E test results with four duplicate strains to which the sites were blinded were closely correlated. With clarithromycin, 39 of 41 (95%) agar dilution and 27 of 31 (87%) E test pairs yielded results within 2 log2 dilutions. With metronidazole, 39 of 44 (89%) agar dilution test pairs and 28 of 32 (88%) E test pairs were within 2 log2 dilutions. The correlation (r2) between the averages of all agar dilution results was 0.9866, and r2 between the averages of all E test values was 0.9396.
For individual sites the results for clarithromycin within 2 log2 dilutions of the final MIC ranged from 69 to 100% (average, 90%; standard deviation [SD], 0.6 to 2.8%; average SD, 1.4%) for the agar dilution method and from 65 to 100% (average, 87%; SD, 1.0 to 2.9%; average SD, 1.7%) for E test. For metronidazole, results within 2 log2 dilutions ranged from 50 to 100% (average, 83%; SD, 0.7 to 3.7%; average SD, 2.0%) for agar dilution and from 50 to 93% (average, 75%; SD, 1.8 to 3.3%; average SD, 2.1%) for E test (Table 1). Site 2 accounted for 10 of the 32 errors and did not complete 25 of 34 E tests because of contamination. When the results from that site were excluded, the agreement by method increased by 3 to 5% and the overall agreement increased to 87%. SDs for the average MICs were slightly greater for E test than for agar dilution for both clarithromycin and metronidazole and were slightly greater for metronidazole than for clarithromycin for both methods (Table 1).
TABLE 1.
Comparison of all agar dilution and E test results for clarithromycin and metronidazole by site and method
| Drug, methodb | Avg % agreement by site ± SDc
|
% Agreement by method | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| CLA, AD | 94 ± 0.9 | 69 ± 2.8 | 76 ± 1.3 | 100 ± 1.3 | 80 ± 2.5 | 100 ± 0.6 | 100 ± 0.9 | 100 ± 0.8 | 90 ± 1.4 |
| CLA, ETd | 100 ± 1.6 | 67 ± 2.5 | 65 ± 1.6 | 100 ± 1.0 | 72 ± 2.9 | 93 ± 1.2 | 85 ± 1.0 | 94 ± 1.7 | 85 ± 1.7 |
| MET, AD | 88 ± 1.5 | 50 ± 3.7 | 94 ± 1.6 | 88 ± 2.2 | 80 ± 2.4 | 73 ± 2.1 | 100 ± 0.7 | 94 ± 1.5 | 83 ± 2.0 |
| MET, ETd | 72 ± 1.8 | 50 ± 3.2 | 94 ± 2.0 | 53 ± 3.3 | 87 ± 2.1 | 93 ± 1.8 | 92 ± 2.0 | 56 ± 2.4 | 75 ± 2.1 |
| % Agreement by sitea | 89 ± 1.6 | 59 ± 3.0 | 82 ± 1.7 | 85 ± 1.7 | 80 ± 2.4 | 90 ± 1.5 | 94 ± 1.0 | 86 ± 1.6 | 83 ± 1.8 |
Sites frequently underestimated MICs by E test relative to the MICs obtained by agar dilution. Site 2 had 10 errors and failed to complete 14 of 17 E tests with clarithromycin and 11 of 17 E tests with metronidazole. When the results from site 2 were excluded from the analysis, all agreements by method increased by 3 to 5% and the overall agreement by site and method increased to 87%.
CLA, claritnromycin; AD, agar diluation method; MET, metronidazole; ET, E test.
Percentage of results equal to or less than the accepted 2 log2 dilutions different from the reference average agar dilution MIC for each H. pylori strain. SD, SDs from the MIC.
E test results compared with reference MICs.
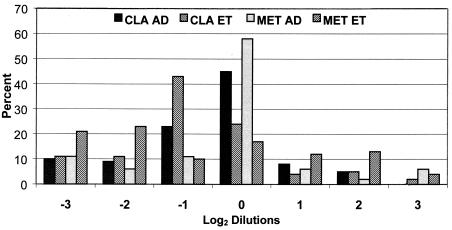
For this study of proficiency testing based on the NCCLS guidelines, the average MIC was derived from the results of the reference agar dilution method, and each agar dilution and E test proficiency testing result was measured against that value. Therefore, the distribution of log2 dilutions relative to the average MIC correlated more closely for the agar dilution method than for E test (Fig. 2). When the individual results obtained by each method were compared with the average MICs for that method, the agreement within 2 log2 dilutions increased from 87 to 88% for clarithromycin by E test and the agreement increased from 72 to 89% for metronidazole by E test (data not shown). No additional errors resulted from this comparison.
FIG. 2.
Percent distribution of clarithromycin (CLA) and metronidazole (MET) log2 dilution MICs by agar dilution (AD) and E test (ET) relative to the average agar dilution MICs for all strains of H. pylori from all sites. A dilution at 0 is equivalent to the average MIC for that strain of H. pylori. Results at −3 and 3 include dilutions equal to and less than or greater than 3 log2 dilutions different from the average, respectively. Compared to the reference agar dilution method, E test MICs tended to be lower than the average, but the numbers of unacceptable results at −3 or 3 were relatively equivalent by all methods.
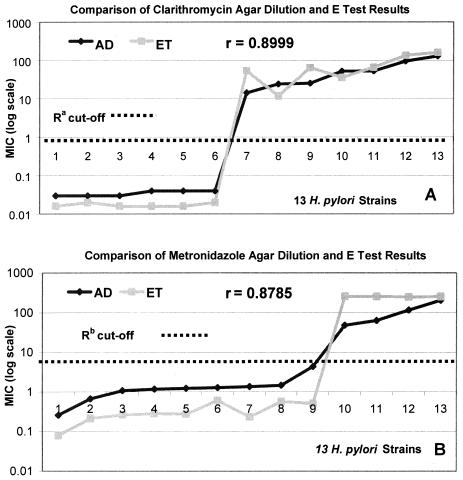
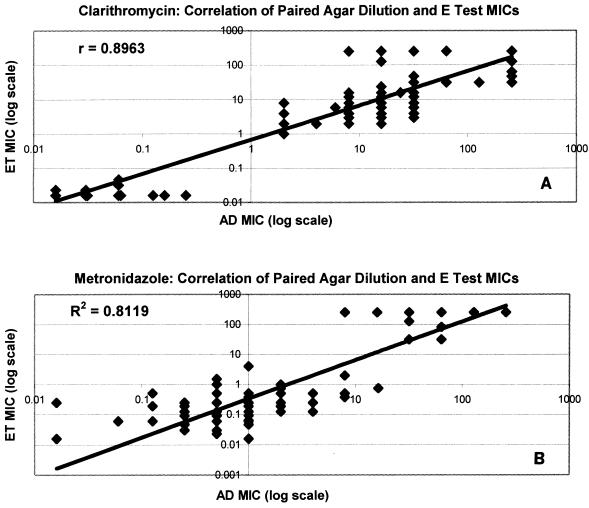
When the average results by agar dilution and E test for each of the 13 strains were compared, it was observed that E test MICs were lower than agar dilution MICs at low values and higher than agar dilution MICs at high values, particularly with metronidazole; but the MICs were consistently in agreement in the susceptibility cutoff range (Fig. 3). For the 13 strains, the correlations (r2) between the average agar dilution MICs and the E test MICs were 0.8999 for clarithromycin and 0.8785 for metronidazole (Fig. 3). When outliers were excluded, the correlations between all paired agar dilution and E test results were 0.8963 for clarithromycin and 0.8112 for metronidazole (Fig. 4). With both antimicrobials and by both methods there was a clear demarcation between susceptible and resistant strains.
FIG. 3.
Comparison of the average of all paired agar dilution (AD) and E test (ET) MICs of clarithromycin (A) and metronidazole (B) for each of the 13 H. pylori strains. Ra, the NCCLS cutoff for clarithromycin resistance is an MIC equal to or greater than 1 μg/ml; Rb, the generally accepted cutoff for metronidazole resistance is an MIC greater than 8 μg/ml. For clarithromycin the overall correlation between the agar dilution method and E test was slightly higher than that for metronidazole, but by both methods with clarithromycin and metronidazole, there was a clear distinction between sensitive and resistant strains.
FIG. 4.
Correlation of E test (ET) and agar dilution (AD) MIC results for all pairs of tests with clarithromycin (A) and metronidazole (B). Values greater than 6 log2 dilutions different from the average MIC by both methods were excluded from the analysis.
DISCUSSION
The clearly demonstrated correlation between antibiotic resistance and treatment failure makes it necessary for decisions about H. pylori therapy to be based on susceptibility test results (2, 11, 19, 22, 35, 37, 42, 44). The aim of the proficiency phase of the Canadian Helicobacter pylori Susceptibility Study was to help establish competence and assess the reproducibility of the results between participating laboratories prior to the beginning of a Canada-wide study of antimicrobial resistance rates. Since the NCCLS guidelines for the testing of H. pylori provide a reference method against which other susceptibility test methods can be measured, the performance of E test relative to that of agar dilution was assessed.
Overall, 93% of all tests correctly identified resistant strains and 83% of all agar dilution and E test results were within 2 log2 dilutions of the average MIC for each strain on the basis of reference agar dilution values. When the results from site 2 were excluded, the overall agreement by method and by site increased from 83 to 87%. The two sites whose results had the lowest correlation with the average MIC results accounted for 18 of 32 (56%) of the VMEs and MEs. Methodology and failure to detect resistant strains, particularly in mixed cultures, caused many of the errors at those sites. In no instance did a value within 2 log2 dilutions of the final MIC cause an error. With the exception of the errors, the results obtained by both tests were acceptable at most sites. Both blinded testing of duplicate strains and unblinded testing in triplicate yielded highly reproducible results by both tests. The reported lack of reproducibility with metronidazole was not evident in these results, possibly because of strict adherence to the methods (14, 34).
There were more VMEs by agar dilution than by E test (13 versus 7) and more MEs by agar dilution than by E test (8 versus 4). Seven VMEs by agar dilution and four VMEs by E test resulted from failure to detect small numbers of resistant colonies. It is more likely that single colonies will be observed on the larger surfaces of E test plates than on the small area covered by the inoculum on agar dilution plates.
In a comparison of the ease of performance of the two methods, for the agar dilution method, most sites reported difficulty with getting antimicrobials into solution, limiting exposure to air during testing, and visualizing growth. E tests were more easily performed. The results on both agar dilution and E test plates must be read by using reflected light to see the very small colonies.
It was observed, particularly with metronidazole, that low E test MICs were generally 2 or 3 log2 dilutions lower than the reference agar dilution MICs, high MICs were often higher by E test, and intermediate MICs most closely agreed by E test (Fig. 3 and 4). However, when E test results were compared with the average E test MIC rather than the average agar dilution MIC, the agreement within 2 log2 dilutions increased from 87 to 88% for clarithromycin and from 75 to 89% for metronidazole. It is possible that the differences in the methods used to get antimicrobials into solution for agar dilution and for E test strips account for these differences. Vaiani et al. (45) used high-pressure liquid chromatography to evaluate the amount of ceftazidime released in agar by E test strips and concluded that antibiotic concentrations, particularly in the intermediate range, were those stated, thus demonstrating the accuracy of E test.
Agar dilution is the “gold standard” method for susceptibility testing of H. pylori, but it is difficult to perform on an individualized basis. E test is easily carried out with small numbers of strains, and although E test strips are expensive, the procedure takes less time than the more labor-intensive agar dilution method. The suitability of E test for H. pylori susceptibility testing, particularly with metronidazole, has been widely debated (13, 14, 34, 46, 48). NCCLS has not published a metronidazole breakpoint for H. pylori, so the E test manufacturer has not been able to seek U.S. Food and Drug Administration approval. However, Japan and several other countries have given approval for the E test method (A. Bolmstrom [AB Biodisk], personal communication). The results from this study show that E test may be a suitable alternative to the agar dilution method. Using standardized protocols and a control strain, the participants in this study correctly identified clarithromycin- and metronidazole-susceptible and -resistant strains of H. pylori 93% of the time using either method. When exact methods were carefully followed, susceptibility results for clarithromycin and metronidazole were highly reproducible by both agar dilution and E test. Therefore, we suggest that agar dilution is useful for studies of large numbers of strains and that E test can be used to discover the susceptibility status of strains from individual patients.
REFERENCES
- 1.Al Qurashi, A. R., F. El Morsy, and A. A. Al Quorain. 2001. Evolution of metronidazole and tetracycline susceptibility pattern in Helicobacter pylori at a hospital in Saudi Arabia. Int. J. Antimicrob. Agents 17:233-236. [DOI] [PubMed] [Google Scholar]
- 2.Bardhan, K., E. Bayerdorffer, S. J. Veldhuyzen van Zanten, T. Lind, F. Megraud, J. C. Delchier, M. Hellblom, A. Stubberod, C. F. Burman, P. Gromark, and L. Zeijlon. 2000. The HOMER Study: the effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter 5:196-201. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoli, F. 2001. Key points from the revised Maastricht Consensus Report: the impact on general practice. Eur. J. Gastroenterol. Hepatol. 13(Suppl. 2):S3-S7. [PubMed] [Google Scholar]
- 4.Bernstein, C. N., I. McKeown, J. M. Embil, J. F. Blanchard, M. Dawood, A. Kabani, E. Kliewer, G. Smart, G. Coghlan, S. Macdonald, C. Cook, and P. Orr. 1999. Seroprevalence of Helicobacter pylori, incidence of gastric cancer, and peptic ulcer-associated hospitalizations in a Canadian Indian population. Dig. Dis. Sci. 44:668-674. [DOI] [PubMed] [Google Scholar]
- 5.Best, L. M., D. J. Haldane, G. S. Bezanson, and S. J. Veldhuyzen van Zanten. 1997. Helicobacter pylori: primary susceptibility to clarithromycin in vitro in Nova Scotia. Can. J. Gastroenterol. 11:298-300. [DOI] [PubMed] [Google Scholar]
- 6.Chaves, S., M. Gadanho, R. Tenreiro, and J. Cabrita. 1999. Assessment of metronidazole susceptibility in Helicobacter pylori: statistical validation and error rate analysis of breakpoints determined by the disk diffusion test. J. Clin. Microbiol. 37:1628-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chida-Sakata, N., M. Baba, H. Inagawa, A. Wada, T. Tanaka, Y. Hoshihara, and T. Takemoto. 1999. Significance of anaerobic preincubation of Helicobacter pylori for measuring metronidazole susceptibility by the Etest. Microbiol. Immunol. 43:397-401. [DOI] [PubMed] [Google Scholar]
- 8.de Wit, N. J., J. Mendive, B. Seifert, F. Cardin, and G. Rubin. 2000. Guidelines on the management of H. pylori in primary care: development of an implementation strategy. Fam. Pract. 17(Suppl. 2):S27-S32. [DOI] [PubMed] [Google Scholar]
- 9.Fallone, C. A. 2000. Epidemiology of the antibiotic resistance of Helicobacter pylori in Canada. Can. J. Gastroenterol. 14:879-882. [DOI] [PubMed] [Google Scholar]
- 10.Fallone, C. A., A. N. Barkun, and E. Halwani. 2001. Does Helicobacter pylori eradication prevent gastric cancer? Dig. Liver Dis. 33:803-804. [DOI] [PubMed] [Google Scholar]
- 11.Fallone, C. A., V. Loo, L. Joseph, J. Barkun, R. Kostyk, and A. N. Barkun. 1999. Predictors of failure of Helicobacter pylori eradication and predictors of ulcer recurrence: a randomized controlled trial. Clin. Investig. Med. 22:185-194. [PubMed] [Google Scholar]
- 12.Franzin, L., M. Pennazio, D. Cabodi, R. F. Paolo, and P. Gioannini. 2000. Clarithromycin and amoxicillin susceptibility of Helicobacter pylori strains isolated from adult patients with gastric or duodenal ulcer in Italy. Curr. Microbiol. 40:96-100. [DOI] [PubMed] [Google Scholar]
- 13.Fukazawa, K., M. Seki, K. Satoh, and K. Sugano. 1999. Antimicrobial resistance testing of H. pylori Epsilometer test and disk diffusion test. Nippon Rinsho 57:76-80. (In Japanese.) [PubMed] [Google Scholar]
- 14.Glupczynski, Y., N. Broutet, A. Cantagrel, L. P. Andersen, T. Alarcon, M. Lopez-Brea, and F. Megraud. 2002. Comparison of the E test and agar dilution method for antimicrobial suceptibility testing of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 21:549-552. [DOI] [PubMed] [Google Scholar]
- 15.Glupczynski, Y., F. Megraud, M. Lopez-Brea, and L. P. Andersen. 2001. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 20:820-823. [DOI] [PubMed] [Google Scholar]
- 16.Grignon, B., J. Tankovic, F. Megraud, Y. Glupczynski, M. O. Husson, M. C. Conroy, J. P. Emond, J. Loulergue, J. Raymond, and J. L. Fauchere. 2002. Validation of diffusion methods for macrolide susceptibility testing of Helicobacter pylori. Microb. Drug Resist. 8:61-66. [DOI] [PubMed] [Google Scholar]
- 17.Grove, D. I., and G. Koutsouridis. 2002. Increasing resistance of Helicobacter pylori to clarithromycin: is the horse bolting? Pathology 34:71-73. [DOI] [PubMed] [Google Scholar]
- 18.Hartzen, S. H., L. P. Andersen, A. Bremmelgaard, H. Colding, M. Arpi, J. Kristiansen, T. Justesen, F. Espersen, N. Frimodt-Moller, and O. Bonnevie. 1997. Antimicrobial susceptibility testing of 230 Helicobacter pylori strains: importance of medium, inoculum, and incubation time. Antimicrob. Agents Chemother. 41:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heep, M., M. Kist, S. Strobel, D. Beck, and N. Lehn. 2000. Secondary resistance among 554 isolates of Helicobacter pylori after failure of therapy. Eur. J. Clin. Microbiol. Infect. Dis. 19:538-541. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen, T. H., A. Lia, R. Schoyen, T. Thoresen, and A. Berstad. 2000. Assessment of optimal atmospheric conditions for growth of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 19:718-720. [DOI] [PubMed] [Google Scholar]
- 21.Hunt, R. H., C. A. Fallone, A. B. Thomson, et al. 1999. Canadian Helicobacter pylori Consensus Conference update: infections in adults. Can. J. Gastroenterol. 13:213-217. [DOI] [PubMed] [Google Scholar]
- 22.Hunt, R. H., F. M. Smaill, C. A. Fallone, P. M. Sherman, S. J. Veldhuyzen van Zanten, A. B. Thomson, et al. 2000. Implications of antibiotic resistance in the management of Helicobacter pylori infection. Can. J. Gastroenterol. 14:862-868. [DOI] [PubMed] [Google Scholar]
- 23.Hunt, R. H., K. Sumanac, and J. Q. Huang. 2001. Review article: should we kill or should we save Helicobacter pylori? Aliment. Pharmacol. Ther. 15(Suppl. 1):51-59. [DOI] [PubMed] [Google Scholar]
- 24.King, A. 2001. Recommendations for susceptibility tests on fastidious organisms and those requiring special handling. J. Antimicrob. Chemother. 48(Suppl. 1):77-80. [DOI] [PubMed] [Google Scholar]
- 25.Leung, W. K., and D. Y. Graham. 2001. Ulcer and gastritis. Endoscopy 33:8-15. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Brea, M., M. J. Martinez, D. Domingo, and T. Alarcon. 2001. A 9 year study of clarithromycin and metronidazole resistance in Helicobacter pylori from Spanish children. J. Antimicrob. Chemother. 48:295-297. [DOI] [PubMed] [Google Scholar]
- 27.Megraud, F., N. Lehn, T. Lind, E. Bayerdorffer, C. O'Morain, R. Spiller, P. Unge, S. V. van Zanten, M. Wrangstadh, and C. F. Burman. 1999. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob. Agents Chemother. 43:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer, J. M., N. P. Silliman, W. Wang, N. Y. Siepman, J. E. Sugg, D. Morris, J. Zhang, H. Bhattacharyya, E. C. King, and R. J. Hopkins. 2002. Risk factors for Helicobacter pylori resistance in the United States: the Surveillance of H. pylori Antimicrobial Resistance Partnership (SHARP) study, 1993-1999. Ann. Intern. Med. 136:13-24. [DOI] [PubMed] [Google Scholar]
- 29.Morgner, A., C. Thiede, E. Bayerdorffer, B. Alpen, T. Wundisch, A. Neubauer, and M. Stolte. 2001. Long-term follow-up of gastric MALT lymphoma after H. pylori eradication. Curr. Gastroenterol. Rep. 3:516-522. [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing and approved standard M7-A5. Informational supplement M100-S10.22. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.Nomura, A. M., G. I. Perez-Perez, J. Lee, G. Stemmermann, and M. J. Blaser. 2002. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am. J. Epidemiol. 155:1054-1059. [DOI] [PubMed] [Google Scholar]
- 32.O'Morain, C., and S. Montague. 2000. Challenges to therapy in the future. Helicobacter 5(Suppl. 1):S23-S26. [DOI] [PubMed] [Google Scholar]
- 33.Osato, M. S. 2000. Antimicrobial susceptibility testing for Helicobacter pylori: sensitivity test results and their clinical relevance. Curr. Pharm. Des. 6:1545-1555. [DOI] [PubMed] [Google Scholar]
- 34.Osato, M. S., R. Reddy, S. G. Reddy, R. L. Penland, and D. Y. Graham. 2001. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int. J. Antimicrob. Agents 17:39-44. [DOI] [PubMed] [Google Scholar]
- 35.Perri, F., M. R. Villani, V. Festa, M. Quitadamo, and A. Andriulli. 2001. Predictors of failure of Helicobacter pylori eradication with the standard ′Maastricht triple therapy.' Aliment. Pharmacol. Ther. 15:1023-1029. [DOI] [PubMed] [Google Scholar]
- 36.Piccolomini, R., G. Di Bonaventura, G. Catamo, F. Carbone, and M. Neri. 1997. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J. Clin. Microbiol. 35:1842-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Realdi, G., M. P. Dore, A. Piana, A. Atzei, M. Carta, L. Cugia, A. Manca, B. M. Are, G. Massarelli, I. Mura, A. Maida, and D. Y. Graham. 1999. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter 4:106-112. [DOI] [PubMed] [Google Scholar]
- 38.Russmann, H., K. Adler, R. Haas, B. Gebert, S. Koletzko, and J. Heesemann. 2001. Rapid and accurate determination of genotypic clarithromycin resistance in cultured Helicobacter pylori by fluorescent in situ hybridization. J. Clin. Microbiol. 39:4142-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman, P., E. Hassall, R. H. Hunt, C. A. Fallone, S. J. Veldhuyzen van Zanten, and A. B. Thomson. 1999. Canadian Helicobacter Study Group Consensus Conference on the approach to Helicobacter pylori infection in children and adolescents. Can. J. Gastroenterol. 13:553-559. [DOI] [PubMed] [Google Scholar]
- 40.Simala-Grant, J. L., D. Zopf, and D. E. Taylor. 2001. Antibiotic susceptibility of attached and free-floating Helicobacter pylori. J. Antimicrob. Chemother. 47:555-563. [DOI] [PubMed] [Google Scholar]
- 41.Smaill, F. 2000. Antibiotic susceptibility and resistance testing: an overview. Can. J. Gastroenterol. 14:871-875. [DOI] [PubMed] [Google Scholar]
- 42.Tankovic, J., D. Lamarque, C. Lascols, C. J. Soussy, and J. C. Delchier. 2001. Clarithromycin resistance of Helicobacter pylori has a major impact on the efficacy of the omeprazole-amoxicillin-clarithromycin therapy. Pathol. Biol. (Paris) 49:528-533. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, D. E., Q. Jiang, and R. N. Fedorak. 1998. Antibiotic susceptibilities of Helicobacter pylori strains isolated in the province of Alberta. Can. J. Gastroenterol. 12:295-298. [DOI] [PubMed] [Google Scholar]
- 44.Tompkins, D. S., J. Perkin, and C. Smith. 1997. Failed treatment of Helicobacter pylori infection associated with resistance to clarithromycin. Helicobacter 2:185-187. [DOI] [PubMed] [Google Scholar]
- 45.Vaiani, R., C. Arcelloni, B. Comuzzi, G. Gesu, C. Bonato, and R. Paroni. 2000. Evaluation of ceftazidime concentration released in agar from an E test strip. Eur. J. Clin. Microbiol. Infect. Dis. 19:551-554. [DOI] [PubMed] [Google Scholar]
- 46.van der Wouden, E. J., A. de Jong, J. C. Thijs, J. H. Kleibeuker, and A. A. van Zwet. 1999. Subpopulations of Helicobacter pylori are responsible for discrepancies in the outcome of nitroimidazole susceptibility testing. Antimicrob. Agents Chemother. 43:1484-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veldhuyzen van Zanten, S. J., N. Flook, N. Chiba, D. Armstrong, A. Barkun, M. Bradette, A. Thomson, F. Bursey, P. Blackshaw, D. Frail, P. Sinclair, et al. 2000. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. Can. Med. Assoc. J. 162(Suppl. 12):S3-S23. [PMC free article] [PubMed] [Google Scholar]
- 48.von Recklinghausen, G., and R. Ansorg. 1995. Metronidazole susceptibility testing of Helicobacter pylori with the PDM epsilometer test (E test). Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 282:83-85. [DOI] [PubMed] [Google Scholar]
- 49.Warburton-Timms, V. J., and C. A. McNulty. 2001. Role of screening agar plates for in vitro susceptibility testing of Helicobacter pylori in a routine laboratory setting. J. Clin. Pathol. 54:408-411. [DOI] [PMC free article] [PubMed] [Google Scholar]