Abstract
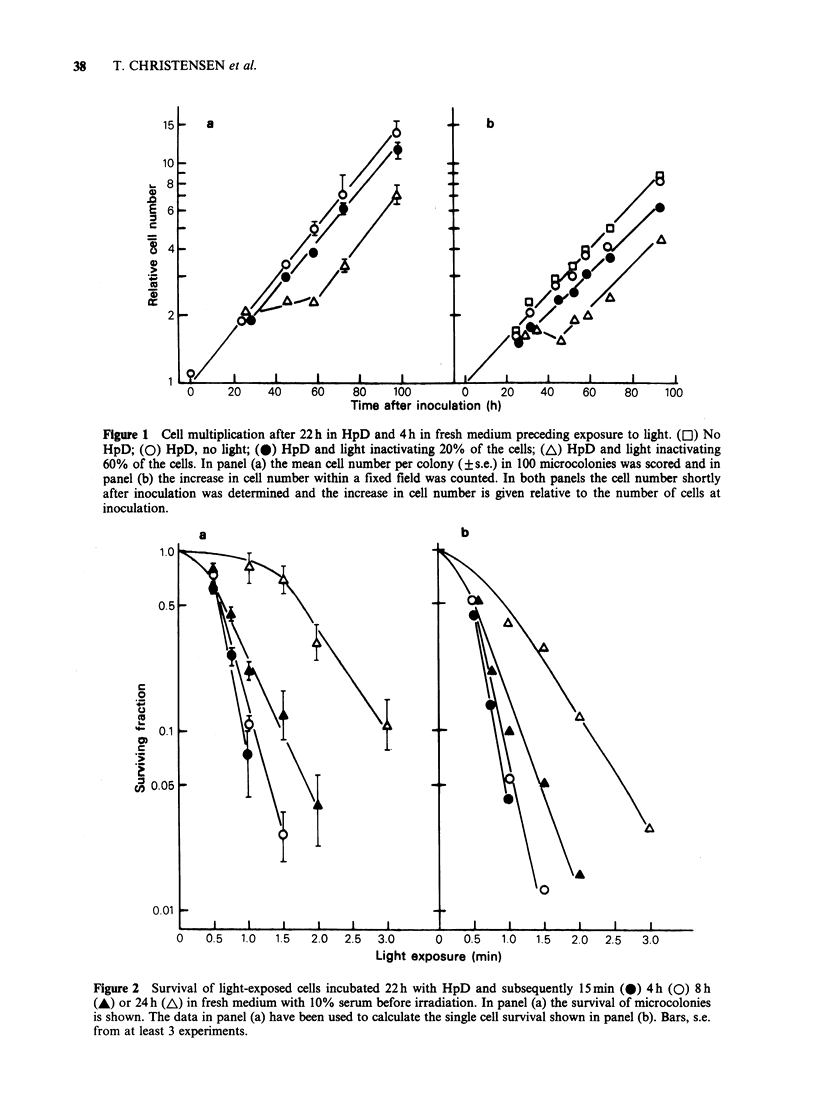
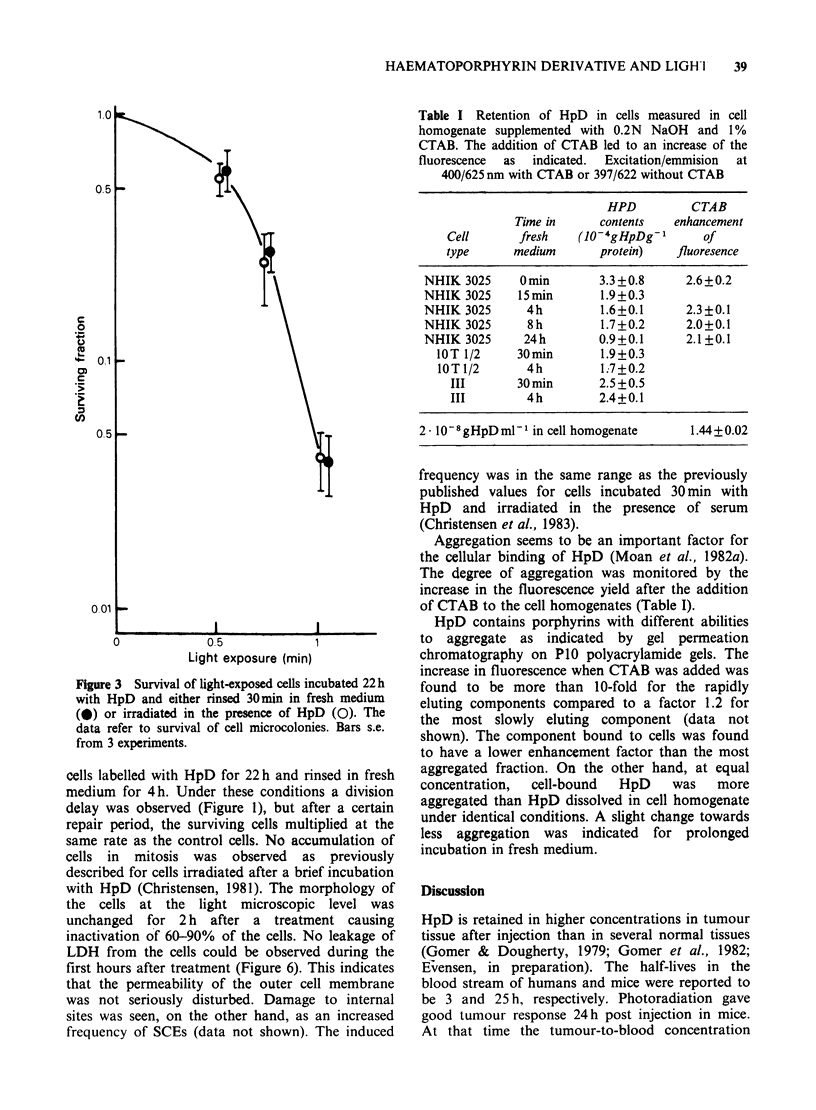
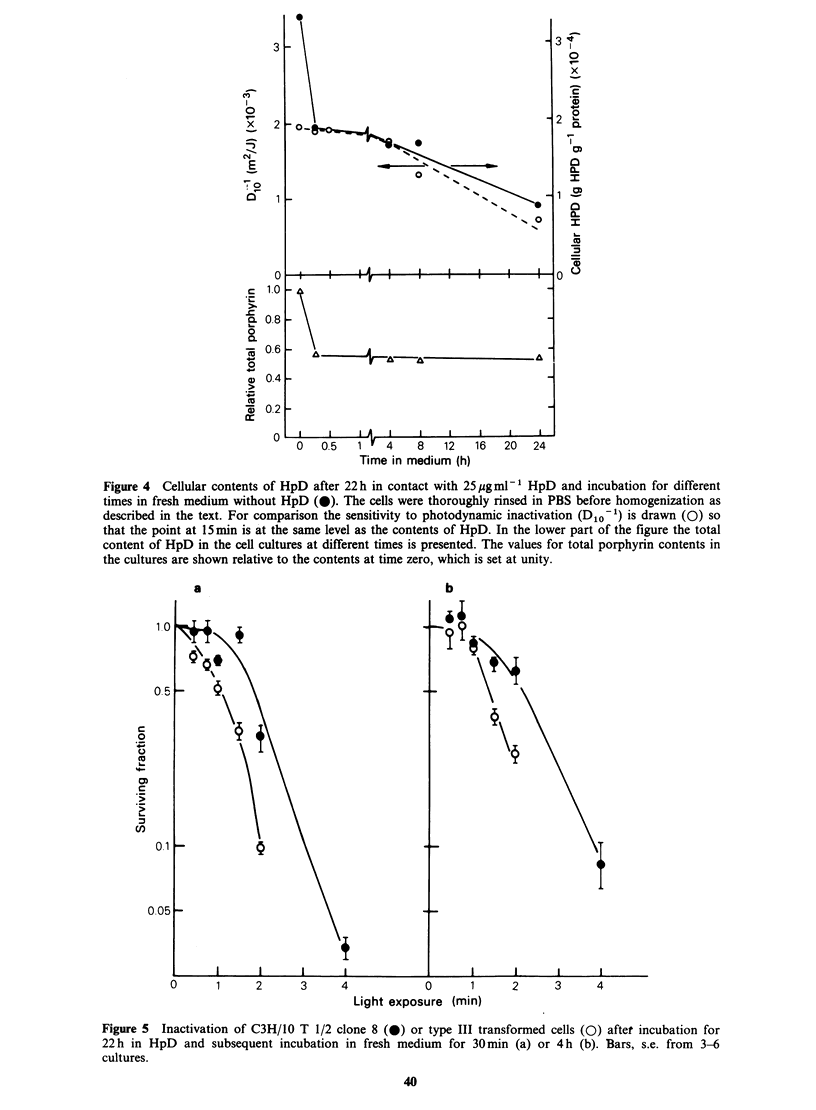
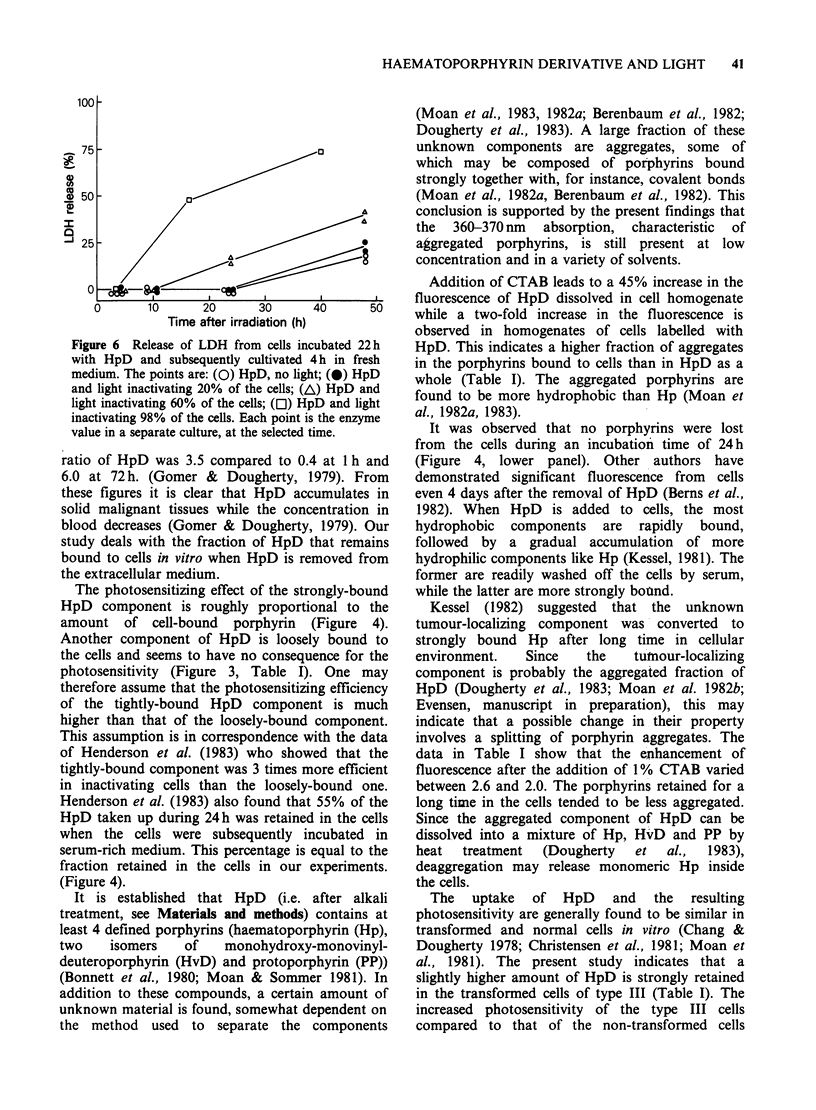
Photoradiation therapy of cancer in the presence of haematoporphyrin derivative is based on a retention of porphyrin in malignant tissue. After long term incubation of NHIK 3025 cells in the presence of 25 microgram ml-1 haematoporphyrin derivative, one fraction is easily removed from the cells by washing with a serum-rich medium. Another fraction remains bound to the cells for a prolonged time. The former does not contribute to the photosensitivity of the cells while the latter, the tightly-bound component, results in a photosensitivity proportional to the cellular contents of porphyrin. Transformed cells are shown to be slightly more sensitive and to retain 25-50% more haematoporphyrin derivative than non-transformed cells. Cytological effects of light absorbed by the tightly-bound component have been studied. The growth of treated cells is similar to that of control cells after a dose-dependent post irradiation lag period. A relatively slow leakage of lactate dehydrogenase (LDH) out of the cells takes place after treatment. The treatment induces a significant increase in the frequency of sister chromatid exchanges (SCE). We conclude that photoactivation of the tightly-bound fraction of haematoporphyrin derivative induces less damage to the outer cell membrane and probably more intracellular damage than irradiation of cells after a short period in contact with the derivative.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellnier D. A., Dougherty T. J. Membrane lysis in Chinese hamster ovary cells treated with hemtoporphyrin derivative plus light. Photochem Photobiol. 1982 Jul;36(1):43–47. doi: 10.1111/j.1751-1097.1982.tb04338.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Bonnett R., Scourides P. A. In vivo biological activity of the components of haematoporphyrin derivative. Br J Cancer. 1982 Apr;45(4):571–581. doi: 10.1038/bjc.1982.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns M. W., Dahlman A., Johnson F. M., Burns R., Sperling D., Guiltinan M., Siemens A., Walter R., Wright W., Hammer-Wilson M. In vitro cellular effects of hematoporphyrin derivative. Cancer Res. 1982 Jun;42(6):2325–2329. [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Christensen T., Feren K., Moan J., Pettersen E. Photodynamic effects of haematoporphyrin derivative on synchronized and asynchronous cells of different origin. Br J Cancer. 1981 Nov;44(5):717–724. doi: 10.1038/bjc.1981.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T. Multiplication of human NHIK 3025 cells exposed to porphyrins in combination with light. Br J Cancer. 1981 Sep;44(3):433–439. doi: 10.1038/bjc.1981.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Volden G., Moan J., Sandquist T. Release of lysosomal enzymes and lactate dehydrogenase due to hematoporphyrin derivative and light irradiation of NHIK 3025 cells in vitro. Ann Clin Res. 1982 Feb;14(1):46–52. [PubMed] [Google Scholar]
- Fritsch P., Gschnait F., Hönigsmann H., Wolff K. Protective action of beta-carotene against lethal photosensitization of fibroblasts in vitro. Br J Dermatol. 1976 Mar;94(3):263–271. doi: 10.1111/j.1365-2133.1976.tb04382.x. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Dougherty T. J. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979 Jan;39(1):146–151. [PubMed] [Google Scholar]
- Gomer C. J., Rucker N., Mark C., Benedict W. F., Murphree A. L. Tissue distribution of 3H-hematoporphyrin derivative in athymic "nude" mice heterotransplanted with human retinoblastoma. Invest Ophthalmol Vis Sci. 1982 Jan;22(1):118–120. [PubMed] [Google Scholar]
- Kessel D. Components of hematoporphyrin derivatives and their tumor-localizing capacity. Cancer Res. 1982 May;42(5):1703–1706. [PubMed] [Google Scholar]
- Kessel D. Effects of photoactivated porphyrins at the cell surface of leukemia L1210 cells. Biochemistry. 1977 Jul 26;16(15):3443–3449. doi: 10.1021/bi00634a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Transport and binding of hematoporphyrin derivative and related porphyrins by murine leukemia L1210 cells. Cancer Res. 1981 Apr;41(4):1318–1323. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Moan J., Christensen T., Sommer S. The main photosensitizing components of hematoporphyrin derivative. Cancer Lett. 1982 Feb;15(2):161–166. doi: 10.1016/0304-3835(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Moan J., Johannessen J. V., Christensen T., Espevik T., McGhie J. B. Porphyrin-sensitized photoinactivation of human cells in vitro. Am J Pathol. 1982 Nov;109(2):184–192. [PMC free article] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J., Sandberg S., Christensen T., Elander S. Hematoporphyrin derivative: chemical composition, photochemical and photosensitizing properties. Adv Exp Med Biol. 1983;160:165–179. doi: 10.1007/978-1-4684-4406-3_16. [DOI] [PubMed] [Google Scholar]
- Moan J., Smedshammer L., Christensen T. Photodynamic effects on human cells exposed to light in the presence of hematoporphyrin. pH effects. Cancer Lett. 1980 Jun;9(4):327–332. doi: 10.1016/0304-3835(80)90025-7. [DOI] [PubMed] [Google Scholar]
- Moan J., Steen H. B., Feren K., Christensen T. Uptake of hematoporphyrin derivative and sensitized photoinactivation of C3H cells with different oncogenic potential. Cancer Lett. 1981 Dec;14(3):291–296. doi: 10.1016/0304-3835(81)90157-9. [DOI] [PubMed] [Google Scholar]
- Sandberg S., Romslo I. Porphyrin-induced photodamage at the cellular and the subcellular level as related to the solubility of the porphyrin. Clin Chim Acta. 1981 Jan 22;109(2):193–201. doi: 10.1016/0009-8981(81)90334-x. [DOI] [PubMed] [Google Scholar]
- Simplicio J., Schwenzer K. Hemin intercalated in micellar cetyltrimethylammonium bromide and triton X-100. A kinetic, spectral, and equilibrium study with cyanide. Biochemistry. 1973 May 8;12(10):1923–1929. doi: 10.1021/bi00734a014. [DOI] [PubMed] [Google Scholar]
- Sinclair W. K., Morton R. A. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res. 1966 Nov;29(3):450–474. [PubMed] [Google Scholar]


