Abstract
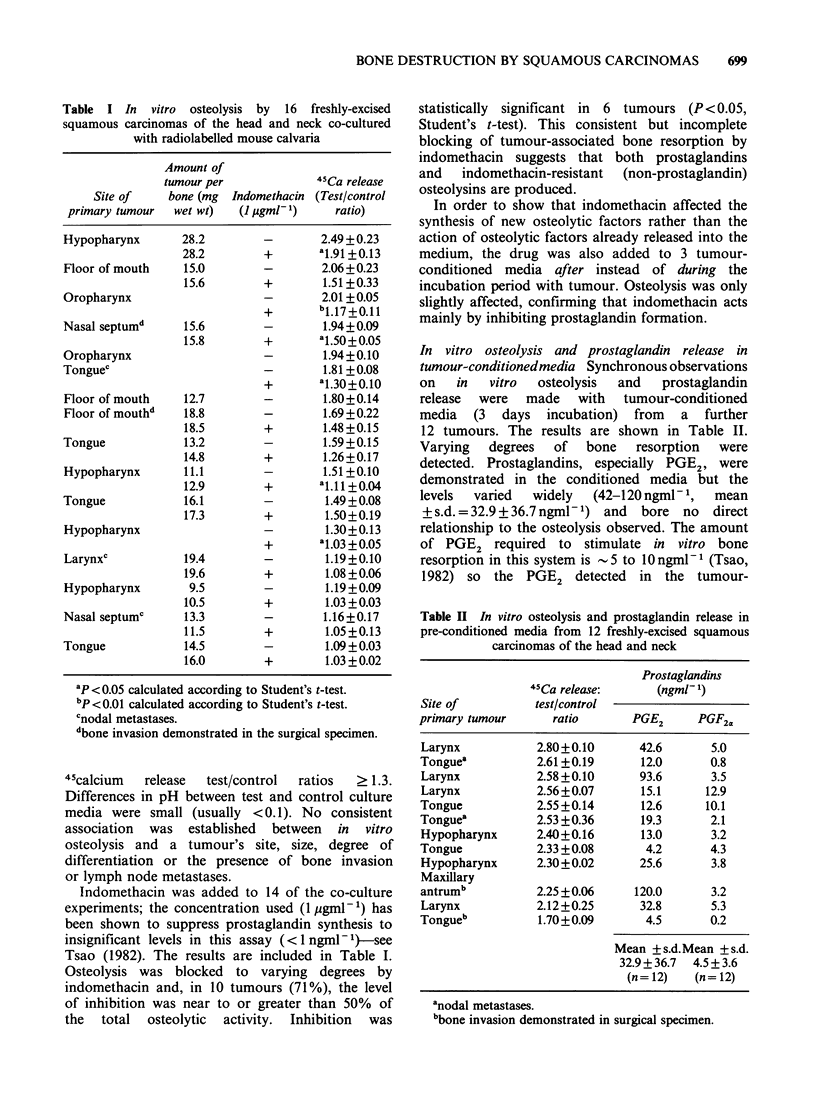
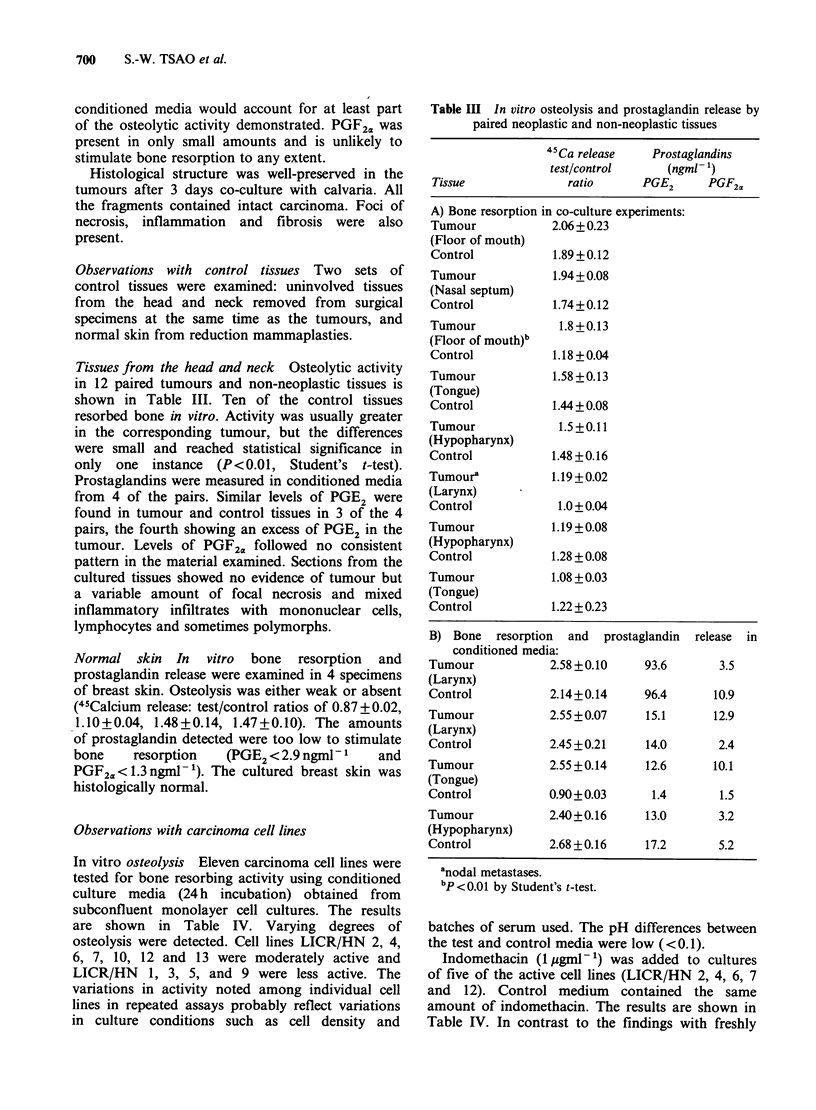
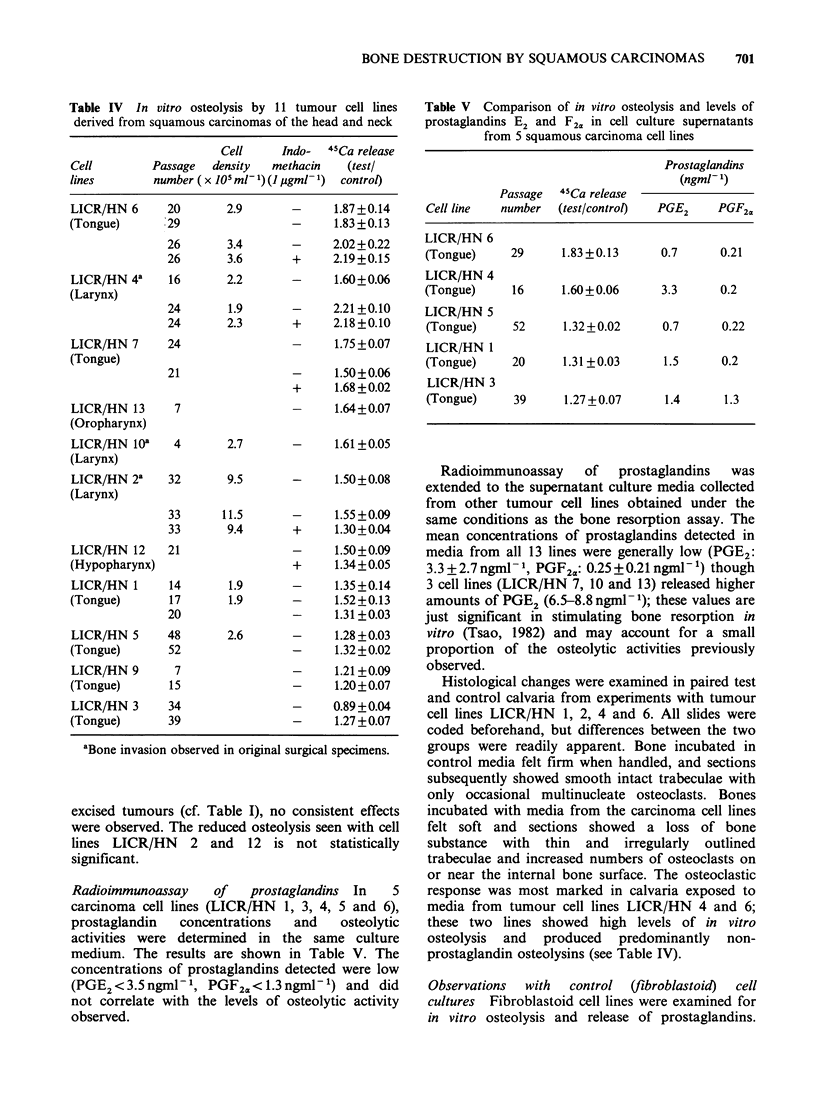
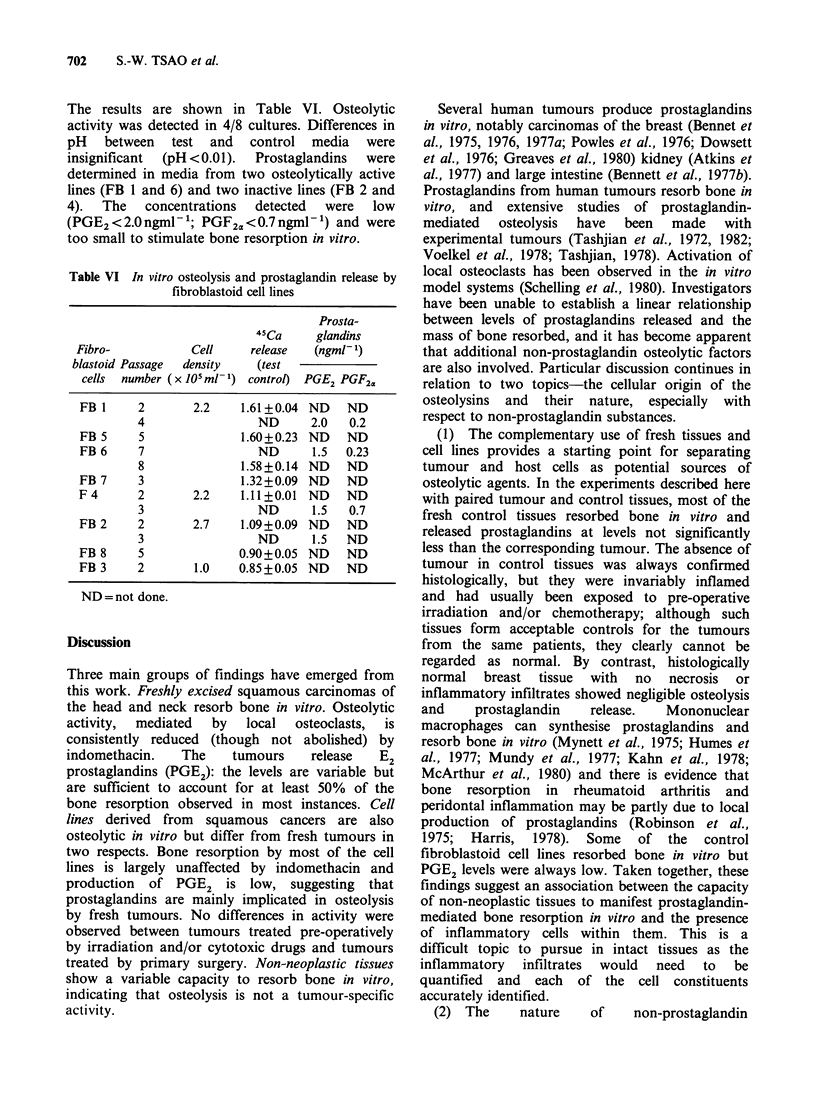
Mechanisms of bone invasion by squamous carcinomas of the head and neck have been investigated using fresh tumours and established tumour cell lines in an in vitro bone resorption assay with 45Ca-labelled mouse calvaria. Fresh tumours regularly resorb bone in vitro. Activity is consistently reduced by indomethacin. The tumours release E2 prostaglandins (PGE2) in amounts sufficient to account for approximately 50% of the bone resorption observed. Small amounts of non-prostaglandin (indomethacin-resistant) osteolytic factors are also produced. Control non-neoplastic tissues show a variable capacity to resorb bone in vitro; PGE2 levels in these tissues may be related to their content of inflammatory cells. Tumour cell lines also resorb bone in vitro but, for most lines, activity is not significantly blocked by indomethacin and PGE2 levels are generally insufficient to account for the osteolysis observed. Non-prostaglandin bone resorbing factors thus predominate. It is concluded that most squamous cancers of the head and neck are osteolytic in vitro and release a mixture of prostaglandin and non-prostaglandin factors which stimulate osteoclastic bone resorption. These factors are derived from both neoplastic and stromal elements, and are "tumour-associated" rather than "tumour-specific". In vitro bone resorption and prostaglandin release does not correlate with pathological features of the tumour or with post-operative survival.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins D., Ibbotson K. J., Hillier K., Hunt N. H., Hammonds J. C., Martin T. J. Secretion of prostaglandins as bone-resorbing agents by renal cortical carcinoma in culture. Br J Cancer. 1977 Nov;36(5):601–607. doi: 10.1038/bjc.1977.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Carter R. L., Stamford I. F., Tanner N. S. Prostaglandin-like material extracted from squamous carcinomas of the head and neck. Br J Cancer. 1980 Feb;41(2):204–208. doi: 10.1038/bjc.1980.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Charlier E. M., McDonald A. M., Simpson J. S., Stamford I. F. Bone destruction by breast tumours. Prostaglandins. 1976 Mar;11(3):461–463. doi: 10.1016/0090-6980(76)90079-4. [DOI] [PubMed] [Google Scholar]
- Bennett A., Charlier E. M., McDonald A. M., Simpson J. S., Stamford I. F., Zebro T. Prostaglandins and breast cancer. Lancet. 1977 Sep 24;2(8039):624–626. doi: 10.1016/s0140-6736(77)92496-5. [DOI] [PubMed] [Google Scholar]
- Bennett A., McDonald A. M., Simpson J. S., Stamford I. F. Breast cancer, prostaglandins, and bone metastases. Lancet. 1975 May 31;1(7918):1218–1220. doi: 10.1016/s0140-6736(75)92197-2. [DOI] [PubMed] [Google Scholar]
- Bennett A., Tacca M. D., Stamford I. F., Zebro T. Prostaglandins from tumours of human large bowel. Br J Cancer. 1977 Jun;35(6):881–884. doi: 10.1038/bjc.1977.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dady P. J., Powles T. J., Dowsett M., Easty G., Williams J., Neville A. M. In vitro osteolytic activity of human breast carcinoma tissue and prognosis. Br J Cancer. 1981 Feb;43(2):222–225. doi: 10.1038/bjc.1981.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M., Easty G. C., Powles T. J., Easty D. M., Neville A. M. Human breast tumour-induced osteolysis and prostaglandins. Prostaglandins. 1976 Mar;11(3):447–460. doi: 10.1016/0090-6980(76)90078-2. [DOI] [PubMed] [Google Scholar]
- Eastman A. R., Dowsett M. The simultaneous separation of individual prostaglandins by thin-layer chromatography on an unmodified support. J Chromatogr. 1976 Nov 17;128(1):224–226. doi: 10.1016/s0021-9673(00)84059-5. [DOI] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Butler L. J. Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck. Br J Cancer. 1981 Jun;43(6):772–785. doi: 10.1038/bjc.1981.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty D. M., Easty G. C., Carter R. L., Monaghan P., Pittam M. R., James T. Five human tumour cell lines derived from a primary squamous carcinoma of the tongue, two subsequent local recurrences and two nodal metastases. Br J Cancer. 1981 Sep;44(3):363–370. doi: 10.1038/bjc.1981.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Stringfellow D. A. Prostaglandin D2 formation by malignant melanoma cells correlates inversely with cellular metastatic potential. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1765–1769. doi: 10.1073/pnas.76.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Ibbotson K. J., Atkins D., Martin T. J. Prostaglandins as mediators of bone resorption in renal and breast tumours. Clin Sci (Lond) 1980 Mar;58(3):201–210. doi: 10.1042/cs0580201. [DOI] [PubMed] [Google Scholar]
- Harris M. Odontogenic cyst growth and prostaglandin-induced bone resorption. Ann R Coll Surg Engl. 1978 Mar;60(2):85–91. [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Josse R. G., Murray T. M., Mundy G. R., Jez D., Heersche J. N. Observations on the mechanism of bone resorption induced by multiple myeloma marrow culture fluids and partially purified osteoclast-activating factor. J Clin Invest. 1981 May;67(5):1472–1481. doi: 10.1172/JCI110177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. J., Stewart C. C., Teitelbaum S. L. Contact-mediated bone resorption by human monocytes in vitro. Science. 1978 Mar 3;199(4332):988–990. doi: 10.1126/science.622581. [DOI] [PubMed] [Google Scholar]
- McArthur W., Yaari A. M., Shapiro I. M. Bone solubilization by mononuclear cells. Lab Invest. 1980 Apr;42(4):450–456. [PubMed] [Google Scholar]
- Mundy C. R., Altman A. J., Gondek M. D., Bandelin J. G. Direct resorption of bone by human monocytes. Science. 1977 Jun 3;196(4294):1109–1111. doi: 10.1126/science.16343. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Luben R. A., Raisz L. G., Oppenheim J. J., Buell D. N. Bone-resorbing activity in supernatants from lymphoid cell lines. N Engl J Med. 1974 Apr 18;290(16):867–871. doi: 10.1056/NEJM197404182901601. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Raisz L. G., Cooper R. A., Schechter G. P., Salmon S. E. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974 Nov 14;291(20):1041–1046. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- Myatt L., Bray M. A., Gordon D., Morley J. Macrophages on intrauterine contraceptive devices produce prostaglandins. Nature. 1975 Sep 18;257(5523):227–228. doi: 10.1038/257227a0. [DOI] [PubMed] [Google Scholar]
- Nimberg R. B., Humphries D. E., Lloyd W. S., Wells H., Schmid K. Purification and partial characterization of a protein from cancer ascites fluid which stimulates the resorption of bone explants in vitro. J Biol Chem. 1982 Mar 10;257(5):2477–2482. [PubMed] [Google Scholar]
- Powles T. J., Dowsett M., Easty G. C., Easty D. M., Neville A. M. Breast-cancer osteolysis, bone metastases, and anti-osteolytic effect of aspirin. Lancet. 1976 Mar 20;1(7960):608–610. doi: 10.1016/s0140-6736(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J. Inhibition by calcitonin of bone resorption induced in vitro by vitamin A. Proc R Soc Lond B Biol Sci. 1968 May 14;170(1018):61–69. doi: 10.1098/rspb.1968.0024. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Tashjian A. H., Jr, Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975 Nov;56(5):1181–1188. doi: 10.1172/JCI108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling S. H., Wolfe H. J., Tashjian A. H., Jr Role of the osteoclast in prostaglandin E2-stimulated bone resorption: a correlative morphometric and biochemical analysis. Lab Invest. 1980 Mar;42(3):290–295. [PubMed] [Google Scholar]
- Tashjian A. H., Jr Role of prostaglandins in the production of hypercalcemia by tumors. Cancer Res. 1978 Nov;38(11 Pt 2):4138–4141. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L., Goldhaber P. Evidence that the bone resorption-stimulating factor produced by mouse fibrosarcoma cells is prostaglandin E 2 . A new model for the hypercalcemia of cancer. J Exp Med. 1972 Dec 1;136(6):1329–1343. doi: 10.1084/jem.136.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S. W., Burman J. F., Carter R. L. Hypercalcaemia and in vitro osteolysis associated with xenografts of squamous carcinomas of the tongue. Br J Cancer. 1983 Jul;48(1):103–107. doi: 10.1038/bjc.1983.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S. W., Burman J. F., Easty D. M., Easty G. C., Carter R. L. Some mechanisms of local bone destruction by squamous carcinomas of the head and neck. Br J Cancer. 1981 Mar;43(3):392–401. doi: 10.1038/bjc.1981.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel E. F., Tashjian A. H., Jr, Franklin R., Wasserman E., Levine L. Hypercalcemia and tumor-prostaglandins: the VX2 carcinoma model in the rabbit. Metabolism. 1975 Aug;24(8):973–986. doi: 10.1016/0026-0495(75)90089-x. [DOI] [PubMed] [Google Scholar]


