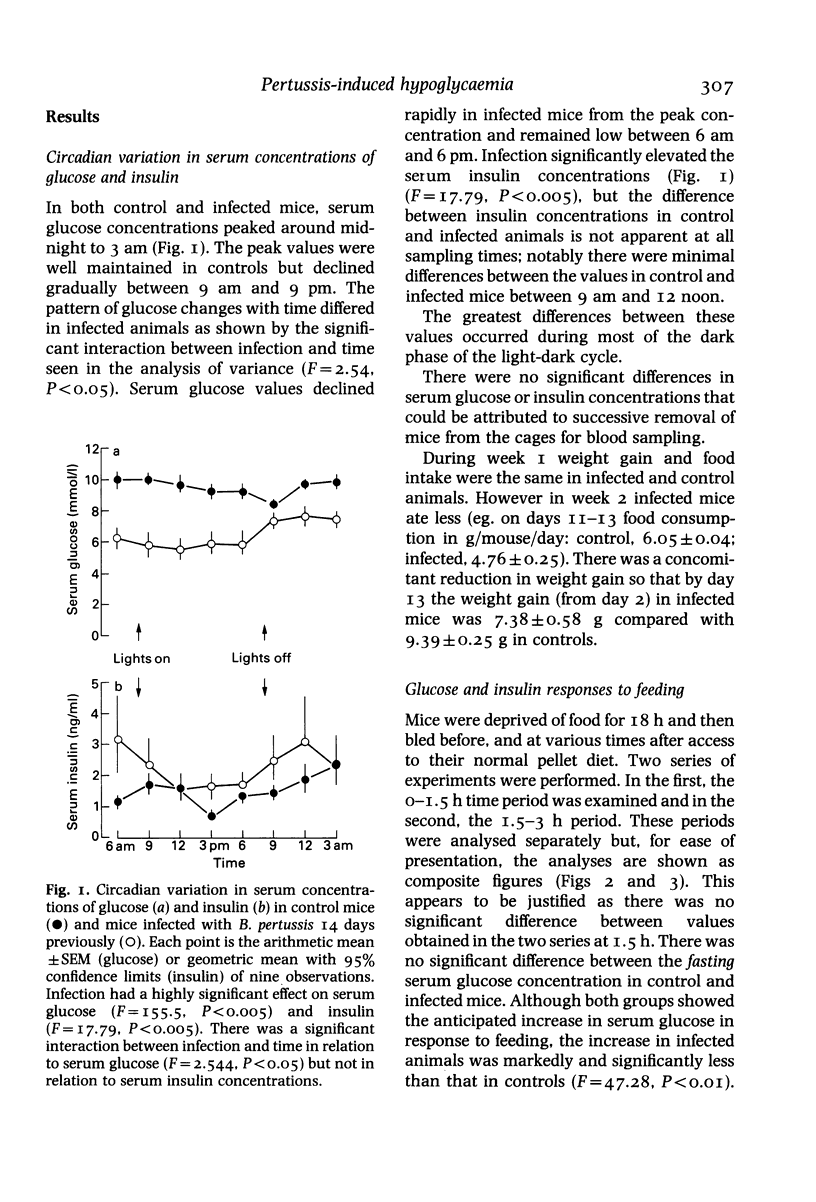
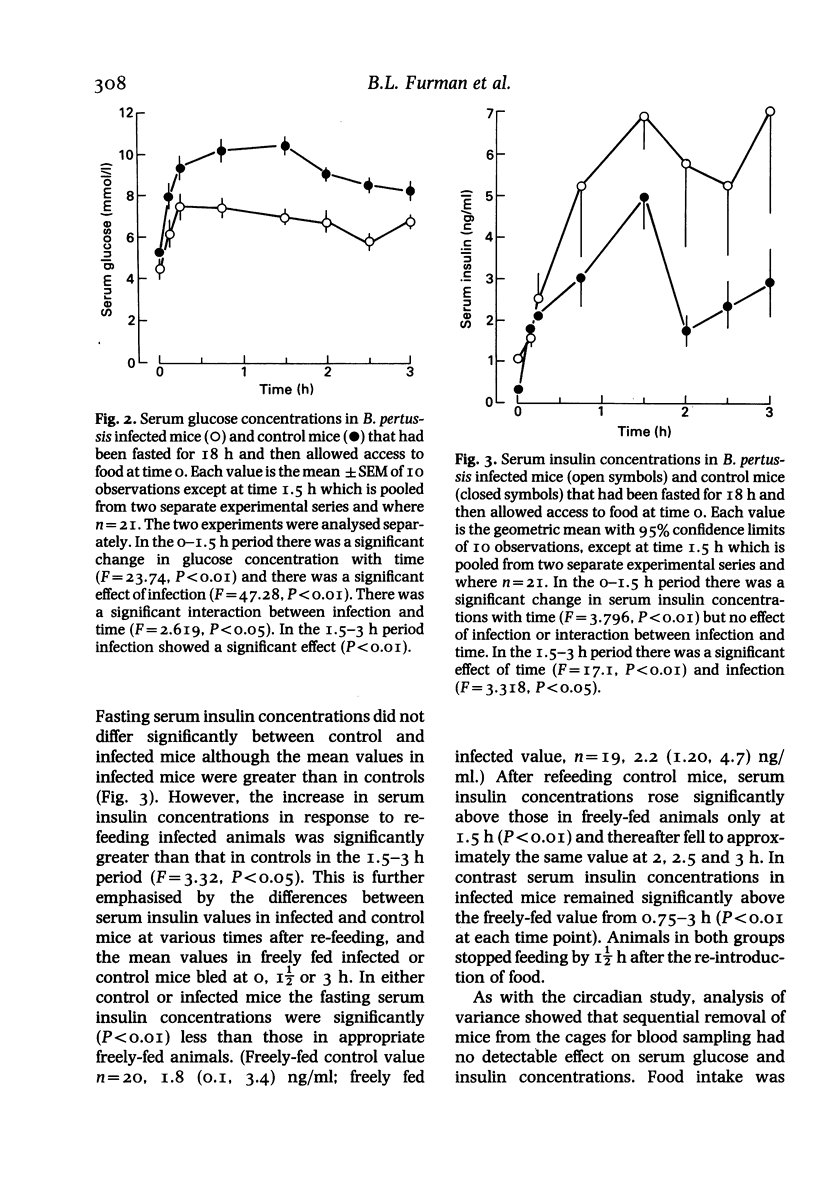
Abstract
Mice were infected intranasally with a sub-lethal dose of Bordetella pertussis organisms (1.2 x 10(5) colony forming units per mouse), control animals receiving the vehicle intranasally. The experiments were performed 14 days later. Serum glucose and insulin concentrations were studied across a 24 h period in freely fed animals and the changes in serum glucose and insulin concentrations in response to feeding were examined in mice fasted for 18 h. The responsiveness of mice to injected insulin (0.5 and 5.0 units/kg i.v.) was also examined. Pertussis-infected mice developed hypoglycaemia and hyperinsulinaemia relative to the controls. These changes were present across a 24 h period although the magnitude of the differences between values seen in control and infected animals varied and the hyperinsulinaemia was not seen at all times. Infected mice showed a markedly diminished hyperglycaemia and an exaggerated hyperinsulinaemia following food ingestion, relative to normal controls. Pertussis-induced hypoglycaemia was abolished following destruction of the pancreatic islet B cells with alloxan (80 mg/kg i.v.). The serum glucose response to a low dose of insulin was significantly attenuated by B. pertussis infection although the hypoglycaemia produced by a high dose was prolonged. It was concluded that B. pertussis infection-induced hypoglycaemia was secondary to hyperinsulinaemia, possibly caused by an exaggerated insulin secretory response to food intake.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Yoshida T., Nakamura N., Koyama K., Nakamura Y., Nakano K., Kondo M. Effect of islet-activating protein (IAP) upon insulin secretion from human pancreatic islets. Endocrinol Jpn. 1981 Apr;28(2):139–143. doi: 10.1507/endocrj1954.28.139. [DOI] [PubMed] [Google Scholar]
- Badr-El-Din M. K., Aref G. H., Mazloum H., El-Towesy Y. A., Kassem A. S., Abdel-Moneim M. A., Abbassy A. A. The beta-adrenergic receptors in pertussis. J Trop Med Hyg. 1976 Oct;79(10):213–217. [PubMed] [Google Scholar]
- Beard J. C., Halter J. B., Best J. D., Pfeifer M. A., Porte D., Jr Dexamethasone-induced insulin resistance enhances B cell responsiveness to glucose level in normal men. Am J Physiol. 1984 Nov;247(5 Pt 1):E592–E596. doi: 10.1152/ajpendo.1984.247.5.E592. [DOI] [PubMed] [Google Scholar]
- Gerich J., Davis J., Lorenzi M., Rizza R., Bohannon N., Karam J., Lewis S., Kaplan R., Schultz T., Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979 Apr;236(4):E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- Gulbenkian A., Schobert L., Nixon, Tabachnick I. I. Metabolic effects of pertussis sensitization in mice and rats. Endocrinology. 1968 Oct;83(4):885–892. doi: 10.1210/endo-83-4-885. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Roberts C. O., Wolff J., Manclark C. R. Biphasic effect of pertussis vaccine on serum insulin in mice. Infect Immun. 1983 Jul;41(1):137–144. doi: 10.1128/iai.41.1.137-144.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A. G. Effect of pertussis vaccination on the sensitivity of mice to insulin, bicarbonate, and CO2. Appl Microbiol. 1969 Jun;17(6):938–939. doi: 10.1128/am.17.6.938-939.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell N. W. Circadian rhythm of glucose tolerance in laboratory mice. Diabetologia. 1970 Oct;6(5):488–492. doi: 10.1007/BF01211889. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Pittman M. The concept of pertussis as a toxin-mediated disease. Pediatr Infect Dis. 1984 Sep-Oct;3(5):467–486. doi: 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Re C. A., Veroni M. C., Larkins R. G. Effect of islet activating protein on glucose tolerance, insulin secretion and insulin responsiveness in the NZO mouse. Diabetologia. 1984 Apr;26(4):304–309. doi: 10.1007/BF00283655. [DOI] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Sen D. K., Arora S., Gupta S., Sanyal R. K. Studies of adrenergic mechanisms in relation to histamine sensitivity in children immunized with Bordetella pertussis vaccine. J Allergy Clin Immunol. 1974 Jul;54(1):25–31. doi: 10.1016/s0091-6749(74)80005-9. [DOI] [PubMed] [Google Scholar]
- Trimble E. R., Weir G. C., Gjinovci A., Assimacopoulos-Jeannet F., Benzi R., Renold A. E. Increased insulin responsiveness in vivo and in vitro consequent to induced hyperinsulinemia in the rat. Diabetes. 1984 May;33(5):444–449. doi: 10.2337/diab.33.5.444. [DOI] [PubMed] [Google Scholar]


