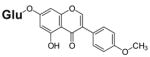
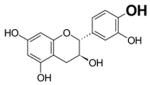
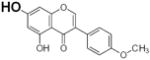
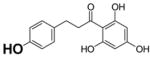
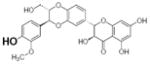
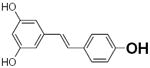
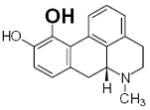
Table 2.
Structure and antioxidant activity of the isoflavones and other polyphenols tested
OH groups with the lowest O-H bond dissociation energy are shown in bold (*for compound 40, the lowest dissociation energy obtained was for the OH group from CH2OH of the carbohydrate residue). N.A., not active; N.D., not determined