Abstract
In this article, a new mechanism influencing the transport of microorganisms through unsaturated porous media is examined, and a new method for directly visualizing bacterial behavior within a porous medium under controlled chemical and flow conditions is introduced. Resting cells of hydrophilic and relatively hydrophobic bacterial strains isolated from groundwater were used as model microorganisms. The degree of hydrophobicity was determined by contact-angle measurements. Glass micromodels allowed the direct observation of bacterial behavior on a pore scale, and three types of sand columns with different gas saturations provided quantitative measurements of the observed phenomena on a porous medium scale. The reproducibility of each break-through curve was established in three to five repeated experiments. The data collected from the column experiments can be explained by phenomena directly observed in the micromodel experiments. The retention rate of bacteria is proportional to the gas saturation in porous media because of the preferential sorption of bacteria onto the gas-water interface over the solid-water interface. The degree of sorption is controlled mainly by cell surface hydrophobicity under the simulated groundwater conditions because of hydrophobic forces between the organisms and the interfaces. The sorption onto the gas-water interface is essentially irreversible because of capillary forces. This preferential and irreversible sorption at the gas-water interface strongly influences the movement and spatial distribution of microorganisms.
Full text
PDF



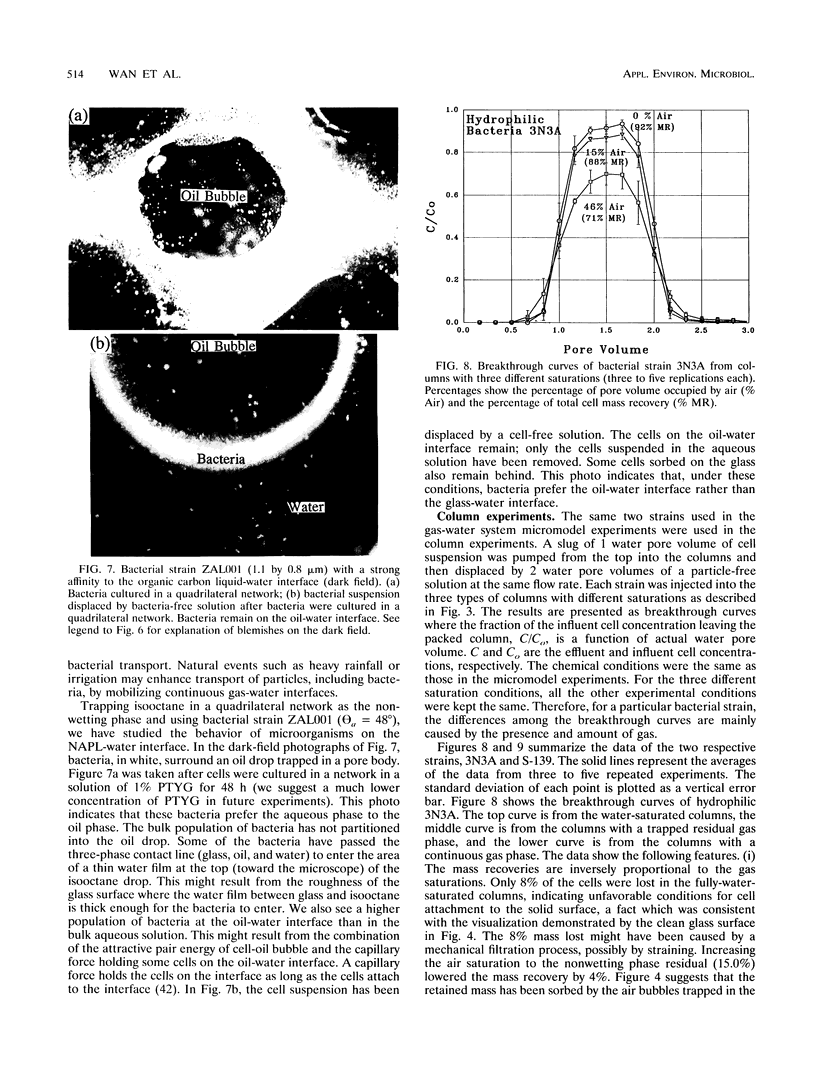
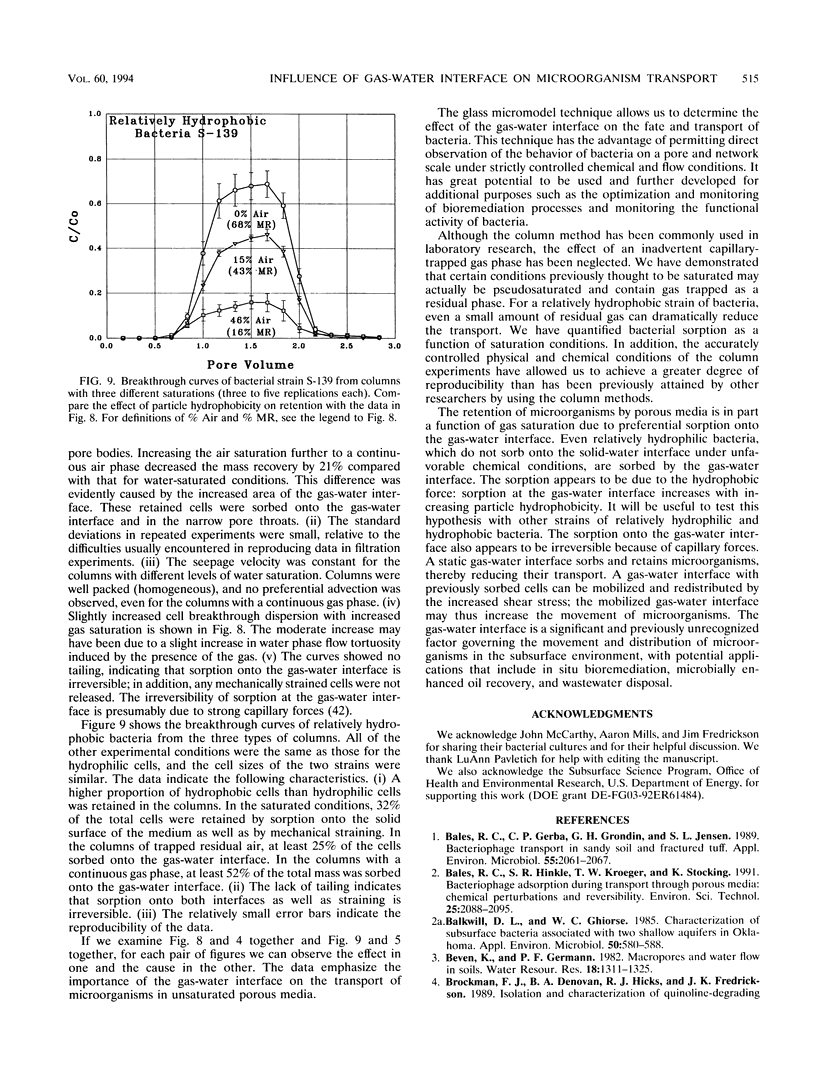

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bales Roger C., Gerba Charles P., Grondin Gerald H., Jensen Stephen L. Bacteriophage Transport in Sandy Soil and Fractured Tuff. Appl Environ Microbiol. 1989 Aug;55(8):2061–2067. doi: 10.1128/aem.55.8.2061-2067.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUS D., WALKER N. THE DECOMPOSITION OF TOLUENE BY SOIL BACTERIA. J Gen Microbiol. 1964 Jul;36:107–122. doi: 10.1099/00221287-36-1-107. [DOI] [PubMed] [Google Scholar]
- Fontes D. E., Mills A. L., Hornberger G. M., Herman J. S. Physical and chemical factors influencing transport of microorganisms through porous media. Appl Environ Microbiol. 1991 Sep;57(9):2473–2481. doi: 10.1128/aem.57.9.2473-2481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhuis J. T., Plugge C. M., Stams A. J., Zehnder A. J. Hydrophobicities and electrophoretic mobilities of anaerobic bacterial isolates from methanogenic granular sludge. Appl Environ Microbiol. 1992 Mar;58(3):1054–1056. doi: 10.1128/aem.58.3.1054-1056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance J. C., Gerba C. P. Virus movement in soil during saturated and unsaturated flow. Appl Environ Microbiol. 1984 Feb;47(2):335–337. doi: 10.1128/aem.47.2.335-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powelson D. K., Simpson J. R., Gerba C. P. Effects of organic matter on virus transport in unsaturated flow. Appl Environ Microbiol. 1991 Aug;57(8):2192–2196. doi: 10.1128/aem.57.8.2192-2196.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981 Oct;148(1):51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- Vandevivere P., Baveye P. Relationship between Transport of Bacteria and Their Clogging Efficiency in Sand Columns. Appl Environ Microbiol. 1992 Aug;58(8):2523–2530. doi: 10.1128/aem.58.8.2523-2530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. M., Landry E. F., Beckwith C. A., Thomas M. Z. Virus removal during groundwater recharge: effects of infiltration rate on adsorption of poliovirus to soil. Appl Environ Microbiol. 1981 Jan;41(1):139–147. doi: 10.1128/aem.41.1.139-147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. V., Gerba C. P., Kelley L. M. Virus persistence in groundwater. Appl Environ Microbiol. 1985 Apr;49(4):778–781. doi: 10.1128/aem.49.4.778-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]