Figure 1.
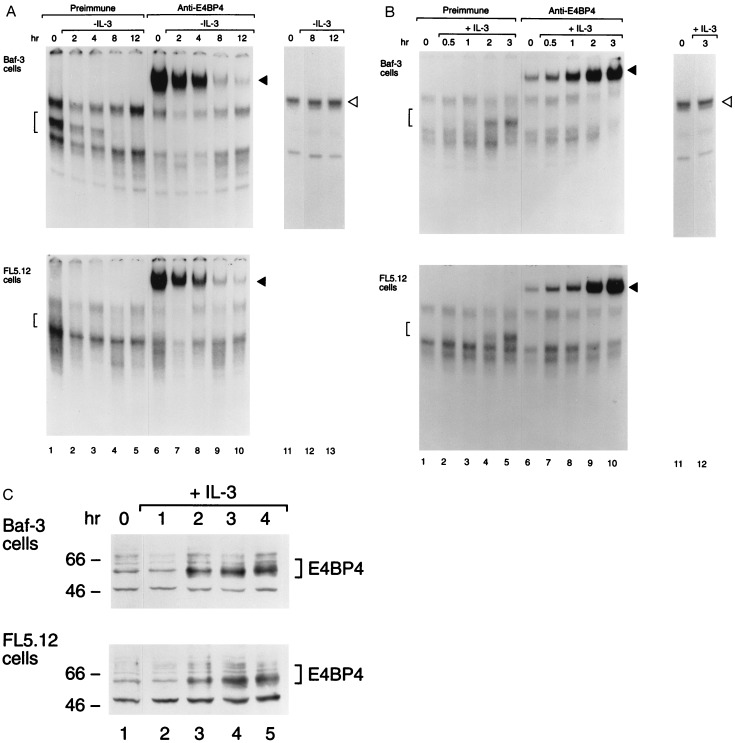
Nfil3/E4bp4 binding activity is regulated by IL-3. (A) Nuclear extracts prepared from IL-3-dependent mouse pro-B cells (Baf-3 or FL5.12) were studied by electrophoretic mobility shift assay with a 32P-labeled oligonucleotide probe containing the HLF consensus binding sequence (GTTACGTAAC; lanes 1–10). Lanes 2–5 and 7–10 show results at the indicated intervals after withdrawal of IL-3 from the medium; lanes 1 and 6 depict findings for stable culture in IL-3. Brackets indicate Nfil3/E4bp4-DNA complexes (lanes 1–5), and solid arrowheads indicate the supershifted complex that was evident after incubation with antiserum specific for Nfil3/E4bp4 (lanes 6–10). As a control for extract integrity, the mobility shift assay was also performed with an SP1 transcription factor consensus sequence probe on extracts from cells growing in IL-3 (lane 11) and after its withdrawal (lanes 12 and 13). (B) Same as in A, except that extracts were analyzed from cells deprived of ligand for 8 hr (lanes 1, 6, and 11) and after the indicated intervals following IL-3 addition (lanes 2–5, 7–10, and 12). (C) Western blot analysis showing levels of the ≈56- to 58-kDa Nfil3/E4bp4 protein in Baf-3 and FL5.12 cells deprived of IL-3 for 8 hr (lane 1) and at 1-hr intervals following reinstatement of the cytokines. Nfil3/E4bp4 protein levels were 4.1- and 4.5-fold higher, respectively, in Baf-3 and FL5.12 cells, 4 hr after IL-3 restimulation (lane 5) than in cells deprived of IL-3 for 8 hr (lane 1). Nfil3/E4bp4 levels in cells growing continuously in IL-3 were 2.5- (Baf-3 cells) and 1.8-fold (FL5.12 cells) higher than in IL-3-deprived cells (lane 1).