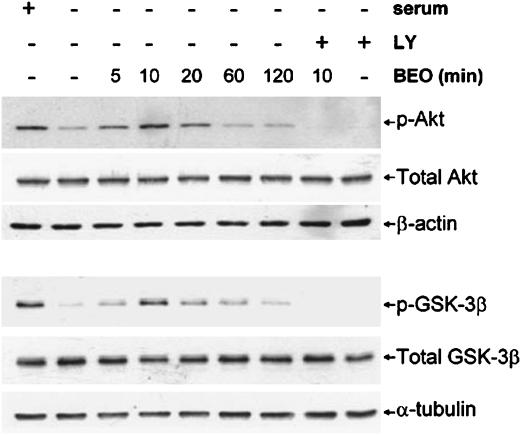
Figure 8.
BEO restored Akt and GSK-3β phosphorylation, reduced by serum withdrawal, via a PI3K-dependent mechanism. SH-SY5Y cells were maintained in serum-containing medium (+serum) or cultured in serum-free medium for 1 h (−serum); serum-starved cells were exposed to 0.01% BEO for 5, 10, 20, 60 and 120 min (min), then cellular proteins were extracted for subsequent analysis by Western blotting of Akt and GSK-3β phosphorylation by using polyclonal antibodies specific for Akt phosphorylated at Ser473 or for GSK-3β phosphorylated at Ser9. Serum deprivation caused a dramatic decline in phospho-Akt (p-Akt) and phospho-GSK-3β (p-GSK-3β) immunoreactivity. Application of BEO for 5–20 min enhanced phosphorylation of Akt and GSK-3β reduced by serum deprivation; this effect peaked at 10 min, then it progressively declined. A preincubation for 30 min with the specific PI3K inhibitor, LY294002 (LY; 20 μM), before exposure to BEO for 10 min, prevented the effects of the essential oil on Akt and GSK-3β phosphorylation. Representative immunoblots from three independent experiments are shown.