Abstract
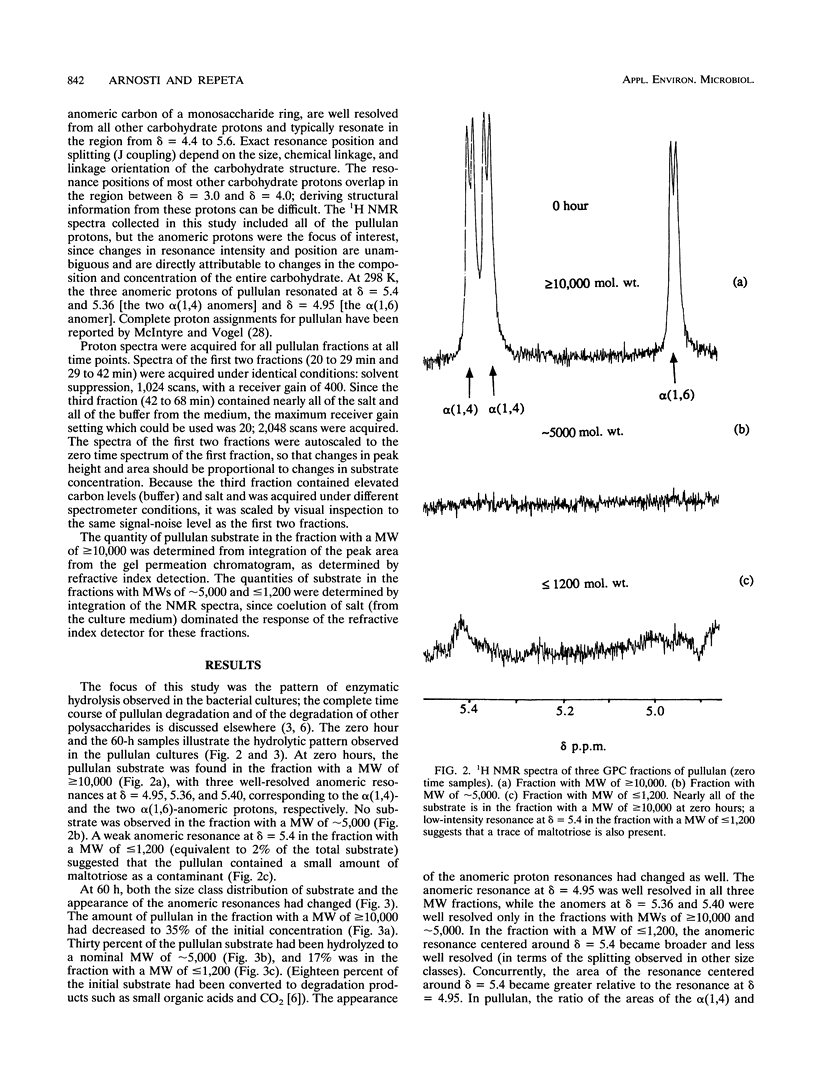
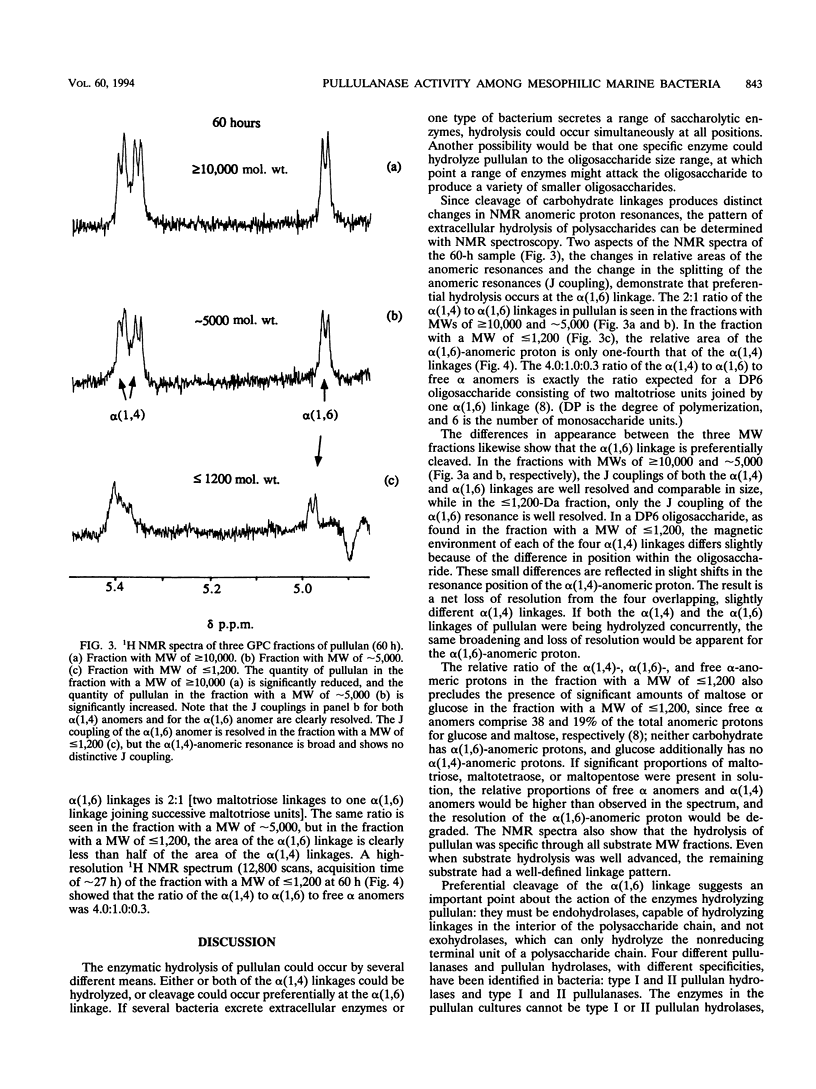
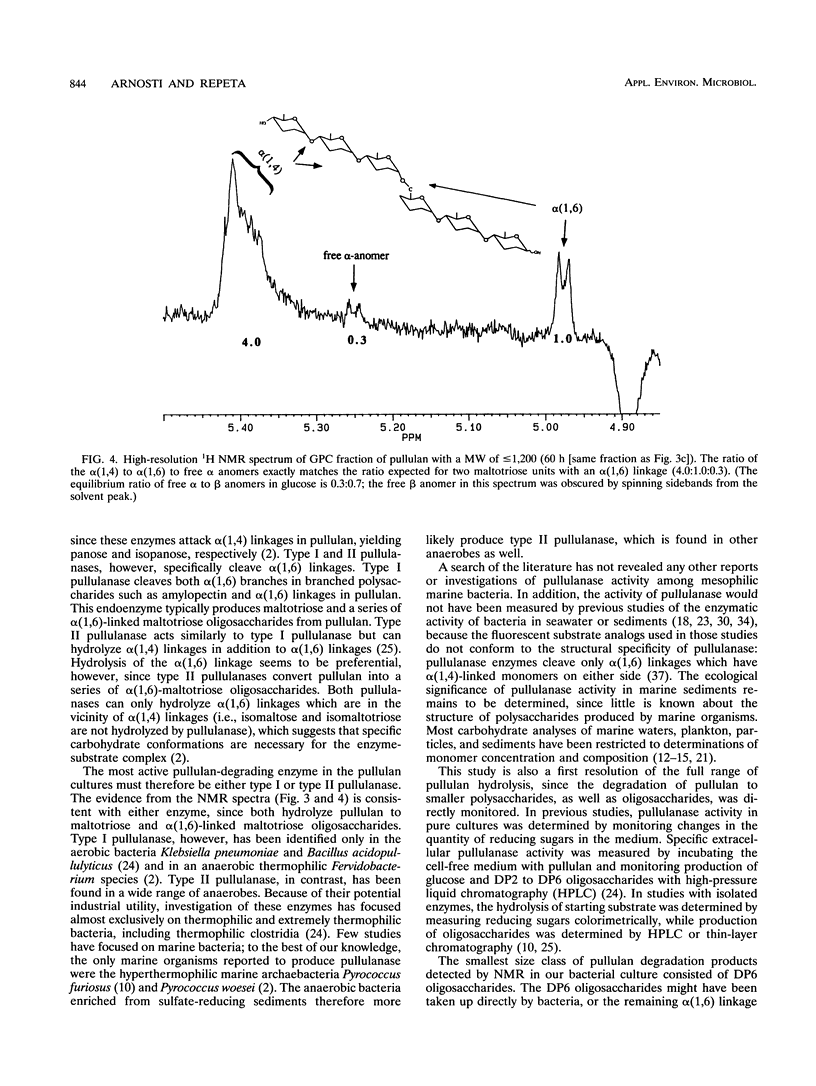
The extracellular enzymatic activity of a mixed culture of anaerobic marine bacteria enriched on pullulan [alpha(1,6)-linked maltotriose units] was directly assessed with a combination of gel permeation chromatography (GPC) and nuclear magnetic resonance spectroscopy (NMR). Hydrolysis products of pullulan were separated by GPC into three fractions with molecular weights of > or = 10,000, approximately 5,000, and < or = 1,200. NMR spectra of these fractions demonstrated that pullulan was rapidly and specifically hydrolyzed at alpha(1,6) linkages by pullulanase enzymes, most likely type II pullulanase. Although isolated pullulanase enzymes have been shown to hydrolyze pullulan completely to maltotriose (S. H. Brown, H. R. Costantino, and R. M. Kelly, Appl. Environ. Microbiol. 56:1985-1991, 1990; M. Klingeberg, H. Hippe, and G. Antranikian, FEMS Microbiol. Lett. 69:145-152, 1990; R. Koch, P. Zablowski, A. Spreinat, and G. Antranikian, FEMS Microbiol. Lett. 71:21-26, 1990), the smallest carbohydrate detected in the bacterial cultures consisted of two maltotriose units linked through one alpha(1,6) linkage. Either the final hydrolysis step was closely linked to substrate uptake, or specialized porins similar to maltoporin might permit direct transport of large oligosaccharides into the bacterial cell. This is the first report of pullulanase activity among mesophilic marine bacteria. The combination of GPC and NMR could easily be used to assess other types of extracellular enzyme activity in bacterial cultures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrykovitch G., Marx I. Isolation of a new polysaccharide-digesting bacterium from a salt marsh. Appl Environ Microbiol. 1988 Apr;54(4):1061–1062. doi: 10.1128/aem.54.4.1061-1062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R. Structure and function of porins from gram-negative bacteria. Annu Rev Microbiol. 1988;42:359–393. doi: 10.1146/annurev.mi.42.100188.002043. [DOI] [PubMed] [Google Scholar]
- Brown B. J., Preston J. F., 3rd L-guluronan-specific alginate lyase from a marine bacterium associated with Sargassum. Carbohydr Res. 1991 Apr 2;211(1):91–102. doi: 10.1016/0008-6215(91)84148-8. [DOI] [PubMed] [Google Scholar]
- Brown S. H., Costantino H. R., Kelly R. M. Characterization of Amylolytic Enzyme Activities Associated with the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1985–1991. doi: 10.1128/aem.56.7.1985-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe Hans-Georg, Kim Sang-Jin, Gocke Klaus. Microbial Decomposition in Aquatic Environments: Combined Process of Extracellular Enzyme Activity and Substrate Uptake. Appl Environ Microbiol. 1988 Mar;54(3):784–790. doi: 10.1128/aem.54.3.784-790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Characterization of beta-Glucosidase Activity in Intertidal Marine Sediments. Appl Environ Microbiol. 1986 Feb;51(2):373–380. doi: 10.1128/aem.51.2.373-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingeberg M., Hippe H., Antranikian G. Production of novel pullulanases at high concentrations by two newly isolated thermophilic clostridia. FEMS Microbiol Lett. 1990 May;57(1-2):145–152. doi: 10.1016/0378-1097(90)90429-t. [DOI] [PubMed] [Google Scholar]
- Meyer-Reil L. A. Seasonal and spatial distribution of extracellular enzymatic activities and microbial incorporation of dissolved organic substrates in marine sediments. Appl Environ Microbiol. 1987 Aug;53(8):1748–1755. doi: 10.1128/aem.53.8.1748-1755.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somville M. Measurement and study of substrate specificity of exoglucosidase activity in eutrophic water. Appl Environ Microbiol. 1984 Dec;48(6):1181–1185. doi: 10.1128/aem.48.6.1181-1185.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]


