Figure 4.
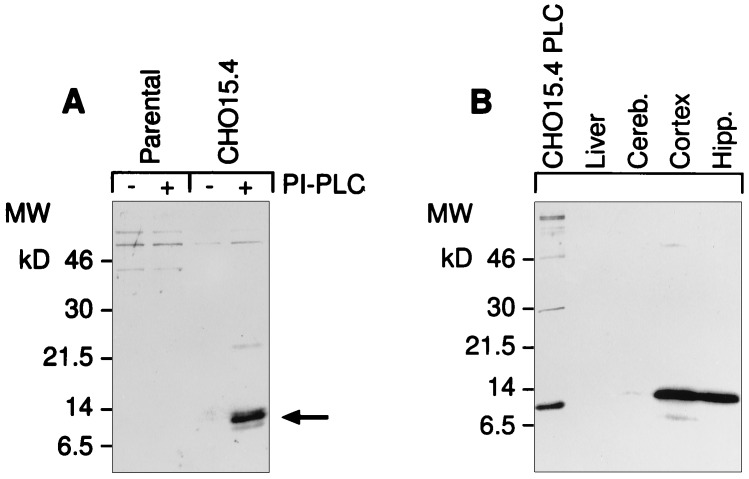
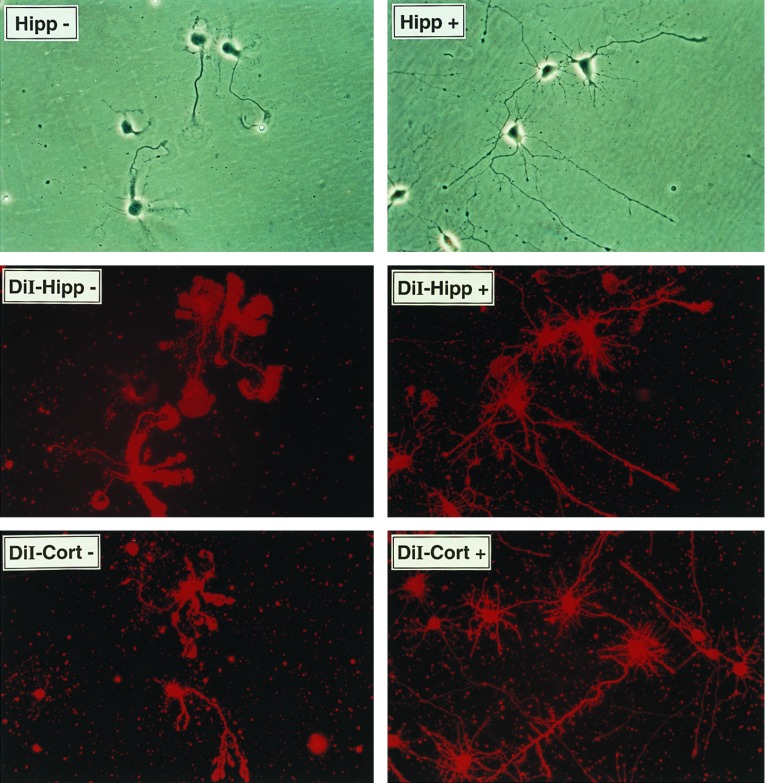
Neuritin is a GPI-anchored protein that promotes neuritogenesis in primary hippocampal and cortical cells. (A) Recombinant human neuritin is sensitive to PI-PLC. Stably transfected CHO cells carrying the neuritin expression vector or the empty parental vector were treated with PI-PLC release buffer alone (−) or in the presence (+) of PI-PLC (0.4 unit/ml). Supernatants were analyzed by probing Western blots with affinity-purified antisera to a synthetic peptide derived from the C-terminal part of mature neuritin. (B) Endogenous neuritin is solubilized in Triton X-114. Tissues were harvested from control or KA-treated rats (i.p., 10 mg/kg, 8 hr) and proteins were extracted by phase separation with Triton X-114. Proteins were analyzed as in A. The first lane is PI-PLC extract from CHO cells expressing recombinant human neuritin (CHO15 cells). The difference in migration is due to the altered mobility of proteins containing an intact GPI anchor (Triton extracts). (C) Histidine-tagged neuritin produced in CHO cells and purified from conditioned media by nickel chromatography promotes neuritogenesis in embryonic hippocampal and cortical cells. Primary neurons from E18 rats were plated at 5 × 103 cells per square centimeter on poly-l-lysine-coated culture dishes in the presence of 150 ng/ml neuritin (+), or a corresponding control nickel-column fraction of parental vector CHO-conditioned media (−). The factor was added at the time of plating and neurite outgrowth was monitored daily. On day 4 of culture, cells were nonspecifically labeled with the lipophilic dye DiI (10 μM in dimethyl sulfoxide) and photographed using phase contrast (Top) or fluorescent (Middle and Bottom) microscopy.