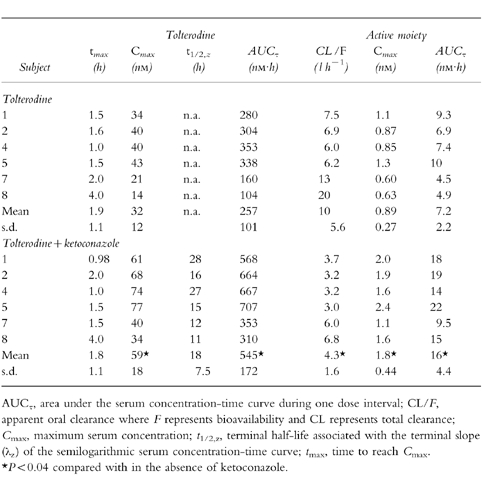
Table 2.
Pharmacokinetic parameters of tolterodine and active moiety (unbound tolterodine) at steady-state during 1 mg twice-daily administration of tolterodine l-tartrate for 2.5 days in the absence and presence of ketoconazole in subjects with deficient CYP2D6 activity.