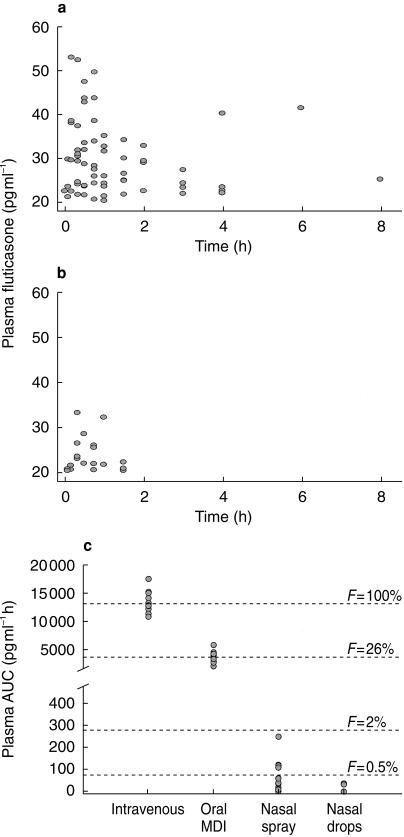
Figure 1.
Individual plasma concentrations of fluticasone propionate in 12 healthy subjects under approximate steady-state conditions during an 8 h dosing interval following (a) 800 µg tds fluticasone propionate aqueous nasal spray or (b) 800 µg tds fluticasone propionate nasal drops. (c) Plasma AUC and absolute bioavailability (F) of intranasal fluticasone propionate relative to intravenous administration and in comparison with oral inhalation (normalized to 800 µg doses, n = 12).