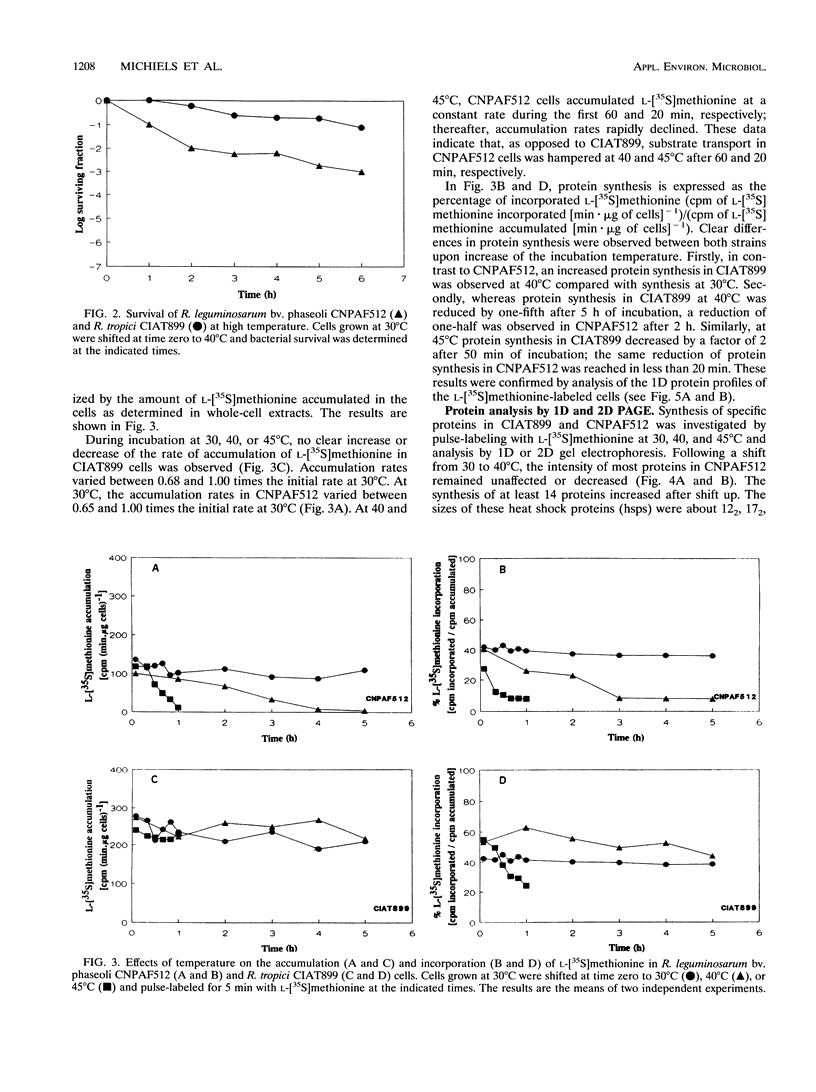
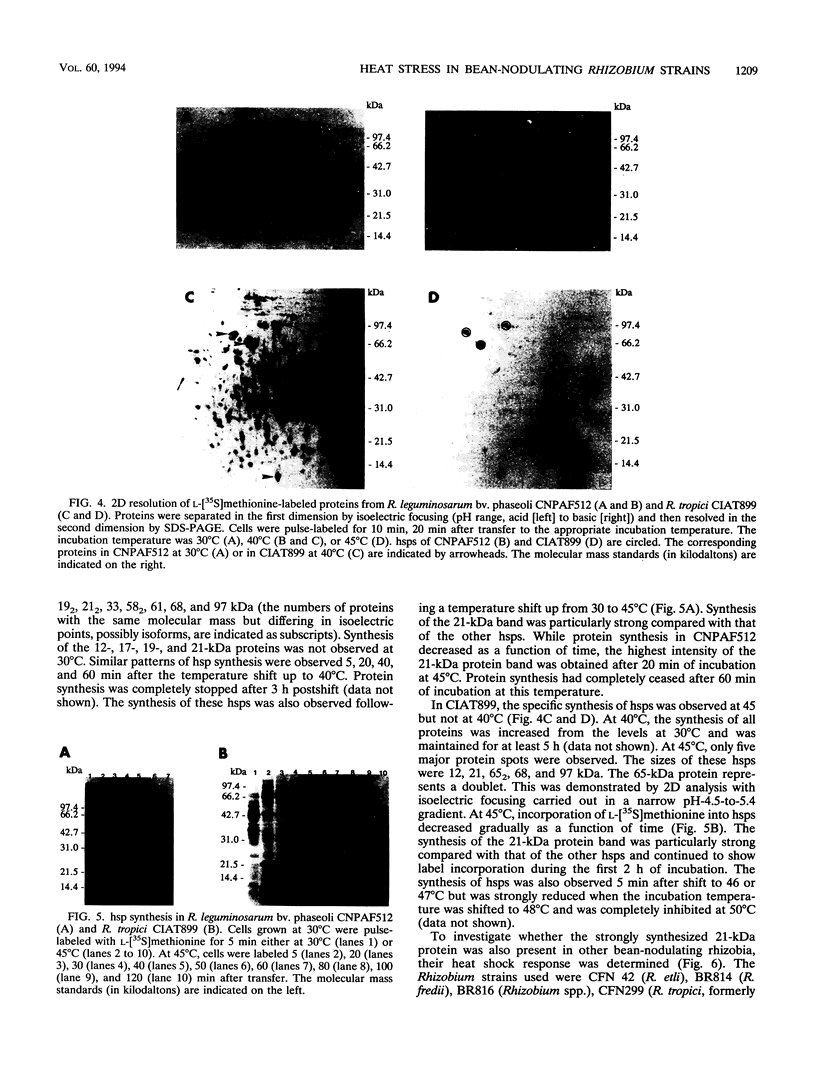
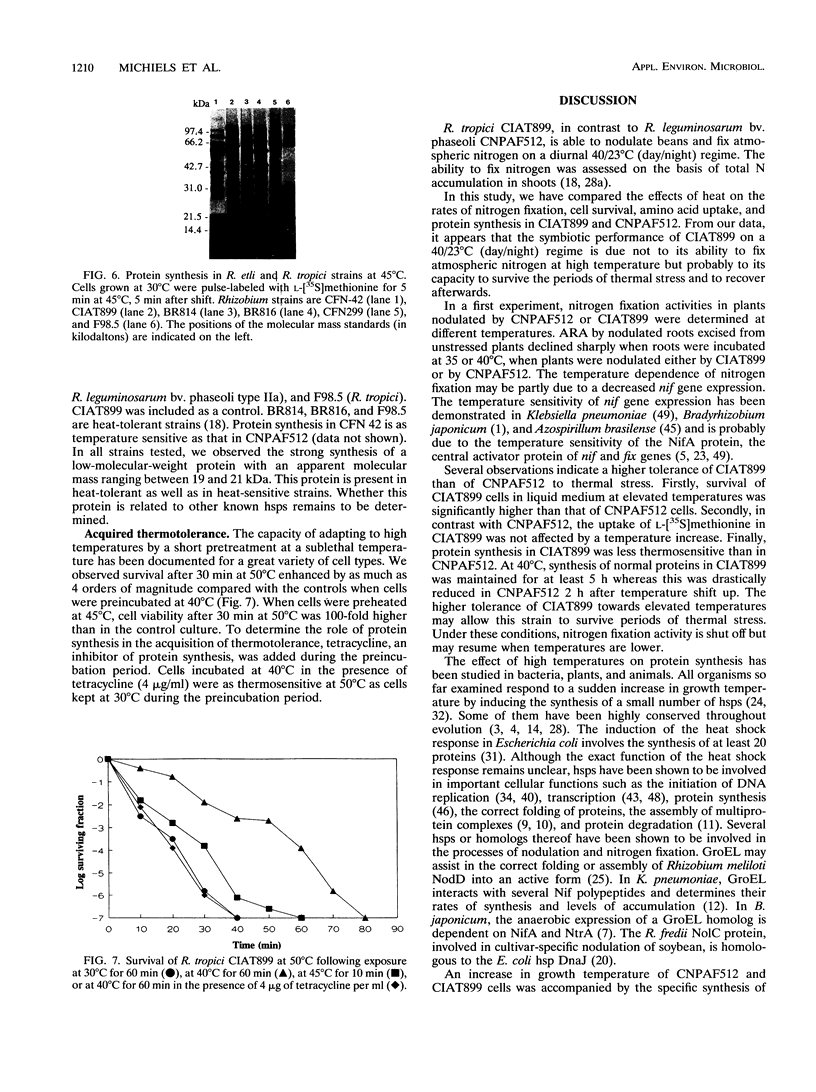
Abstract
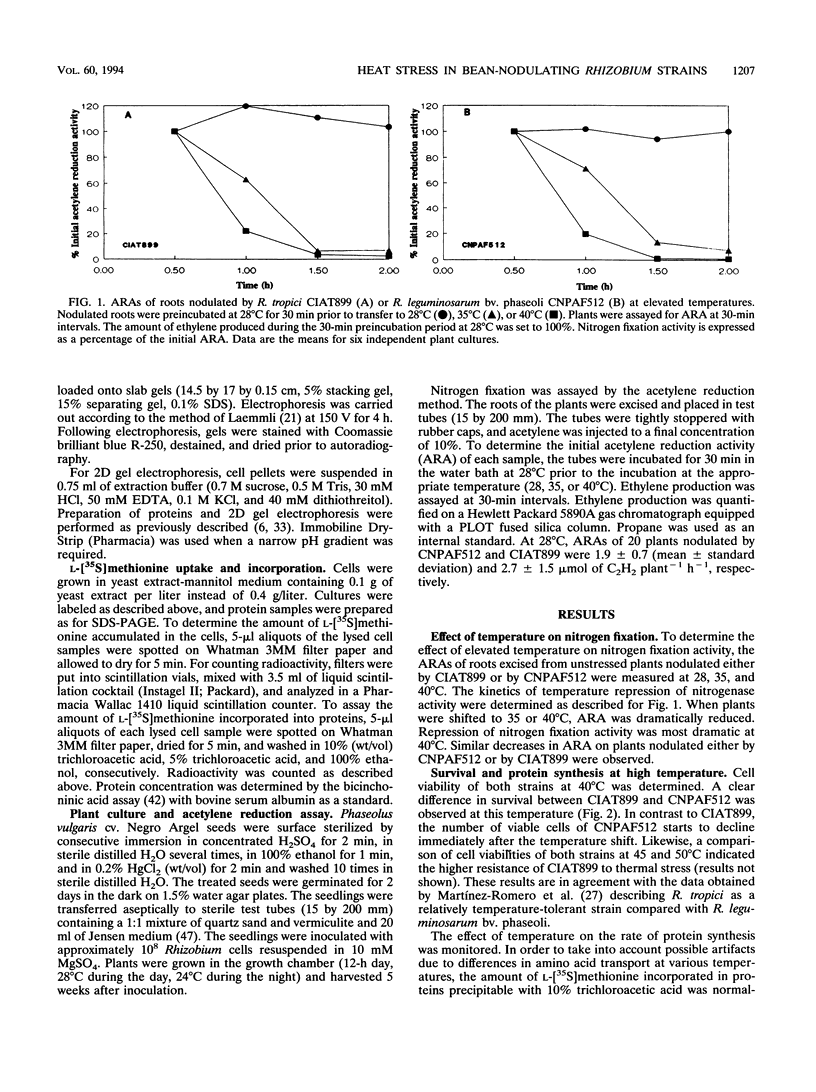
High soil temperatures in tropical areas limit nodulation and dinitrogen fixation by strains of Rhizobium. Several heat-tolerant bean-nodulating Rhizobium strains have been isolated previously. However, the basis of their resistance to heat remains unknown. In this study, we compared the effects of heat on symbiotic nitrogen fixation, cell survival, amino acid uptake, and protein synthesis in a heat-tolerant (CIAT899) and a heat-sensitive (CNPAF512) bean-nodulating Rhizobium strain. Acetylene reduction activity of nodulated roots excised from unstressed plants was strongly diminished at 35 or 40°C when plants were nodulated either by CIAT899 or by CNPAF512. When these strains were tested under free-living conditions, survival at 40°C as well as the kinetics of l-[35S]methionine uptake and protein synthesis at 35 and 40°C indicated the higher tolerance of CIAT899 than of CNPAF512 to thermal stress. The synthesis of heat shock proteins was detected in both strains, although at different temperatures. Increased synthesis of 14 heat shock proteins in CNPAF512 and of 6 heat shock proteins in CIAT899 was observed at 40 and 45°C, respectively. A heat shock protein of approximately 21 kDa, of which the synthesis was strongest in both Rhizobium strains upon a temperature shift up, was also conserved in several other bean-nodulating rhizobia. Acquired thermotolerance in CIAT899 was shown to depend on protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Morales A., Hennecke H. Expression of Rhizobium japonicum nifH and nifDK operons can be activated by the Klebsiella pneumonia nifA protein but not by the product of ntrC. Mol Gen Genet. 1985;199(2):306–314. doi: 10.1007/BF00330273. [DOI] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. J., Collins J. J., Brill W. J. Repression of nitrogen fixation in Klebsiella pneumoniae at high temperature. J Bacteriol. 1984 Feb;157(2):460–464. doi: 10.1128/jb.157.2.460-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. M., Babst M., Kaspar T., Acuña G., Arigoni F., Hennecke H. One member of a gro-ESL-like chaperonin multigene family in Bradyrhizobium japonicum is co-regulated with symbiotic nitrogen fixation genes. EMBO J. 1993 Jul;12(7):2901–2912. doi: 10.1002/j.1460-2075.1993.tb05952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanaris G. A., Papavassiliou A. G., Rubock P., Silverstein S. J., Gottesman M. E. Renaturation of denatured lambda repressor requires heat shock proteins. Cell. 1990 Jun 15;61(6):1013–1020. doi: 10.1016/0092-8674(90)90066-n. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Govezensky D., Greener T., Segal G., Zamir A. Involvement of GroEL in nif gene regulation and nitrogenase assembly. J Bacteriol. 1991 Oct;173(20):6339–6346. doi: 10.1128/jb.173.20.6339-6346.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Krishnan H. B., Pueppke S. G. nolC, a Rhizobium fredii gene involved in cultivar-specific nodulation of soybean, shares homology with a heat-shock gene. Mol Microbiol. 1991 Mar;5(3):737–745. doi: 10.1111/j.1365-2958.1991.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Berger D. K., Kustu S. Activity of purified NIFA, a transcriptional activator of nitrogen fixation genes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2266–2270. doi: 10.1073/pnas.90.6.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Martínez-Romero E., Segovia L., Mercante F. M., Franco A. A., Graham P., Pardo M. A. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol. 1991 Jul;41(3):417–426. doi: 10.1099/00207713-41-3-417. [DOI] [PubMed] [Google Scholar]
- Martínez E., Palacios R., Sánchez F. Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J Bacteriol. 1987 Jun;169(6):2828–2834. doi: 10.1128/jb.169.6.2828-2834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullin T. W., Hallberg R. L. A highly evolutionarily conserved mitochondrial protein is structurally related to the protein encoded by the Escherichia coli groEL gene. Mol Cell Biol. 1988 Jan;8(1):371–380. doi: 10.1128/mcb.8.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J., Vande Broek A., Vanderleyden J. Molecular cloning and nucleotide sequence of the Rhizobium phaseoli recA gene. Mol Gen Genet. 1991 Sep;228(3):486–490. doi: 10.1007/BF00260644. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paek K. H., Walker G. C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987 Jan;169(1):283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possingham J. V., Moye D. V., Anderson A. J. Influence of Elevated Shoot and Root Temperature on Nitrogen Fixation. Plant Physiol. 1964 Jul;39(4):561–563. doi: 10.1104/pp.39.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Atkins C. A., Pate J. S. Effect of temperature on nitrogenase functioning in cowpea nodules. Plant Physiol. 1983 Oct;73(2):392–394. doi: 10.1104/pp.73.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y. The dnaK gene of Escherichia coli functions in initiation of chromosome replication. J Bacteriol. 1988 Feb;170(2):972–979. doi: 10.1128/jb.170.2.972-979.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia L., Young J. P., Martínez-Romero E. Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int J Syst Bacteriol. 1993 Apr;43(2):374–377. doi: 10.1099/00207713-43-2-374. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Toro N., Olivares J. Analysis of Rhizobium meliloti Sym Mutants Obtained by Heat Treatment. Appl Environ Microbiol. 1986 May;51(5):1148–1150. doi: 10.1128/aem.51.5.1148-1150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Vaughn V., Neidhardt F. C. Gene for heat-inducible lysyl-tRNA synthetase (lysU) maps near cadA in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1066–1068. doi: 10.1128/jb.153.2.1066-1068.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Itikawa H. Participation of Escherichia coli K-12 groE gene products in the synthesis of cellular DNA and RNA. J Bacteriol. 1984 Feb;157(2):694–696. doi: 10.1128/jb.157.2.694-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Brill W. J. Temperature sensitivity of the regulation of nitrogenase synthesis by Klebsiella pneumoniae. J Bacteriol. 1981 Feb;145(2):1116–1118. doi: 10.1128/jb.145.2.1116-1118.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkowski W. Molecular mechanism for loss of nodulation properties of Rhizobium trifolii. J Bacteriol. 1982 Jun;150(3):999–1007. doi: 10.1128/jb.150.3.999-1007.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]