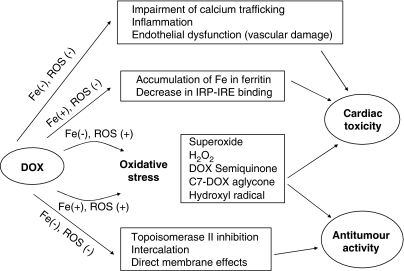
Figure 1.
A simplified scheme of the proposed interactions of doxorubicin with tumour and cardiac cells illustrates their complexity. With regard to iron, the effects of doxorubicin can be divided to Fe-dependent and Fe-independent. The most prominent characteristic of doxorubicin is its ability to induce oxidative stress. ROS can be formed in the absence of Fe (semiquinone radical, C7-aglycone radical, superoxide, H2O2) but their generation can also be Fe-catalyzed (Fenton reaction, Haber–Weiss reaction), creating highly damaging HO• radicals. Doxorubicin (DOX) can also form DOX–Fe(III) complexes that also lead to ROS. ROS may induce damage to both cardiac and neoplastic cells. The higher susceptibility of heart tissue to oxidative stress is often explained by its poor antioxidant defences and/or abundance of mitochondria, which are both important source, and target of ROS. The major mechanisms that lead to cancerous cell death are, most probably, inhibition of topoisomerase II and DNA intercalation effects independent of oxidative stress and iron. Other effects that do not depend on ROS are thought to contribute predominantly to their cardiotoxicity: dysregulation of iron homeostasis via interaction with iron regulatory protein and inhibition of Fe mobilization from ferritin are examples of Fe-mediated effects whereas inflammation, endothelial dysfunction and calcium homeostasis impairment are both iron and ROS independent.