FIGURE 2. Phosphorylation of S. cerevisiae-expressed human CTP synthetase 1 by protein kinase A, and the effect of protein kinase A phosphorylation on CTP synthetase 1 activity.
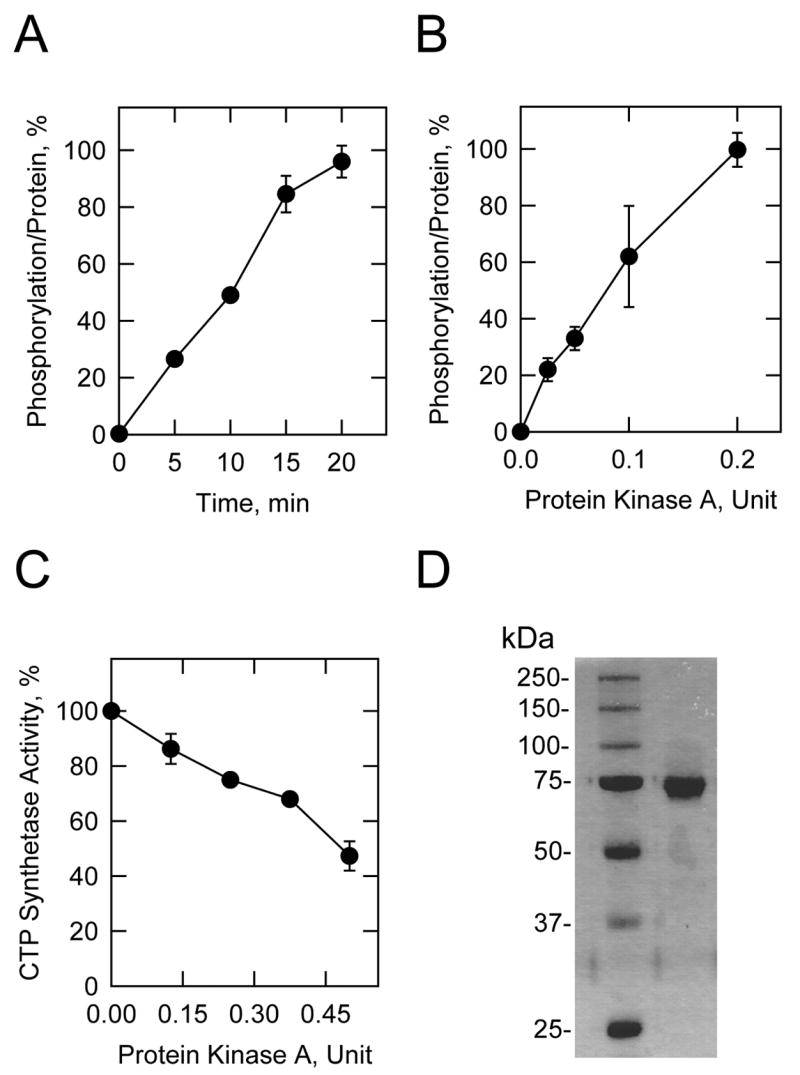
A, purified wild type human CTP synthetase 1 was incubated with mammalian protein kinase A (0.1 U/ml) and [γ-32P]ATP for the indicated time intervals. B, purified wild type human CTP synthetase 1 was incubated with [γ-32P]ATP and the indicated amounts of mammalian protein kinase A for 10 min. After incubations, samples were subjected to SDS-PAGE and transferred to PVDF membrane. The phosphorylated proteins were subjected to phosphorimaging analysis and the relative amounts of phosphate incorporated were quantified using ImageQuant software. The maximum extent of CTP synthetase 1 phosphorylation was set at 100%. The data were normalized to the amount of human CTP synthetase 1 protein as determined by immunoblot analysis using anti-His6 antibodies. The values reported were average of four separate experiments ± S.D. C, purified human CTP synthetase 1 was phosphorylated for 20 min with the indicated amounts of mammalian protein kinase A using unlabeled ATP. The control reaction contained unlabeled ATP and omitted protein kinase A. The product CTP was extracted from the reaction mixture and analyzed by high performance liquid chromatography. The CTP synthetase activity measured without protein kinase A phosphorylation was set at 100%. The values reported were the average of three separate experiments ± S.D. D, purified wild type CTP synthetase 1 was subjected to SDS-PAGE and stained with Coomassie blue. The positions of the protein molecular mass standards are indicated on the left side of the figure.
