FIGURE 4. Effects of the T455A, S552A, and T455A,S552A mutations on the phosphorylation of human CTP synthetase 1 by protein kinase A, and phosphoamino acid analysis of phosphorylated proteins.
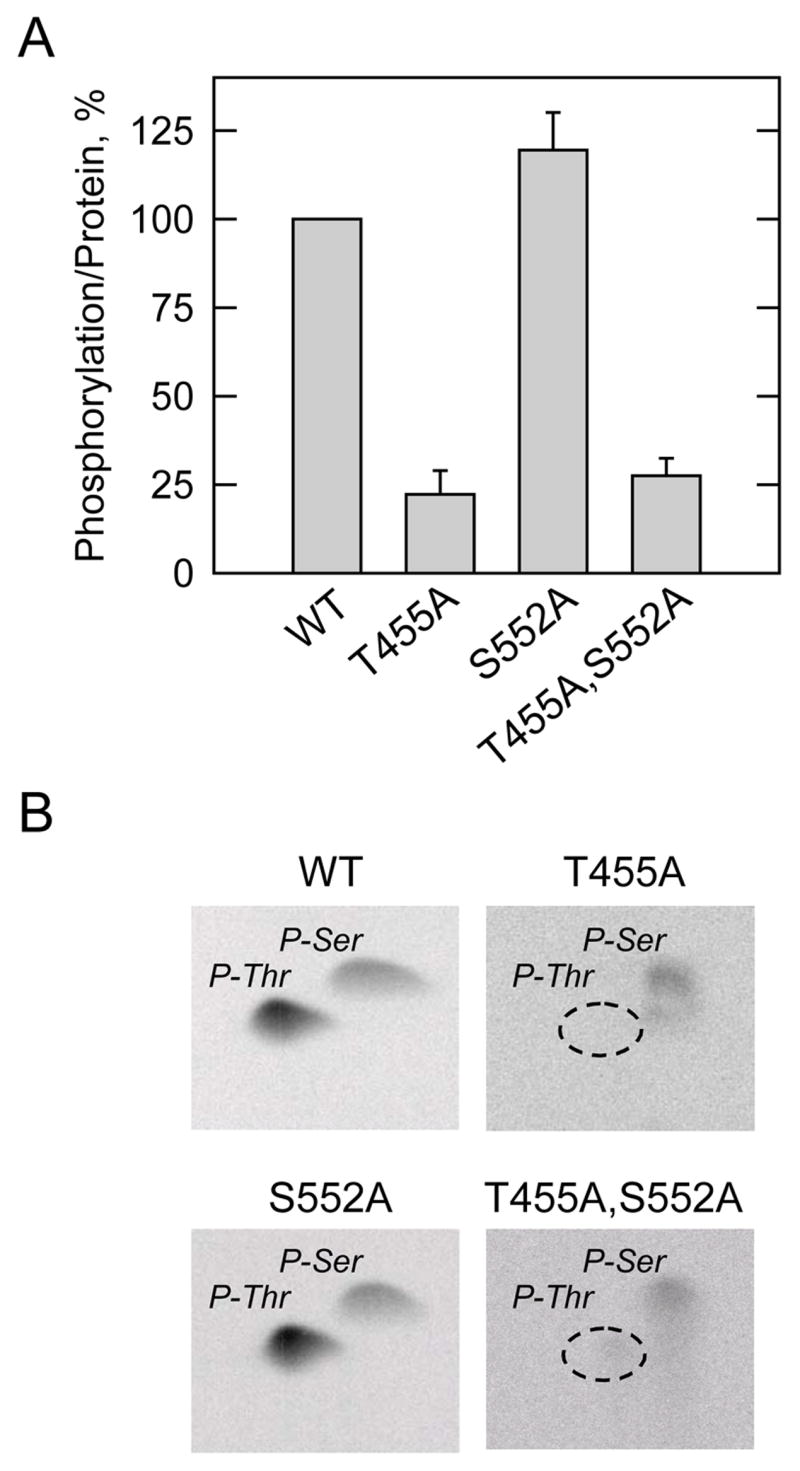
A, the wild type (WT) and the indicated mutant human CTP synthetase 1 enzymes were purified from cell extracts using Ni2+-NTA resin. The enzymes were phosphorylated with mammalian protein kinase A and [γ-32P]ATP for 10 min at 30 °C. After the phosphorylation reactions, the samples were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membrane. The phosphorylated proteins were subjected to phosphorimaging analysis and the relative amounts of phosphate incorporated were quantified using ImageQuant software. The maximum extent of wild type CTP synthetase 1 phosphorylation was set at 100%. The data were normalized to the amount of human CTP synthetase 1 proteins as determined by immunoblot analysis using anti-His6 antibodies. The values reported were average of two separate experiments ± S.D. B, the 32P-labeled wild type and indicated mutant human CTP synthetase 1 enzymes on polyvinylidene difluoride membranes were hydrolyzed with 6N HCl, and the hydrolysates were separated on cellulose thin layer plates by two-dimensional electrophoresis. The positions of the carrier standard phosphoamino acids phosphoserine (P-Ser) and phosphothreonine (P-Thr) are indicated in the figure. The position of phosphothreonine that was absent in the T455A and T455A,S552A mutant enzymes that was present in the wild type and S552A mutant enzymes is indicated in the figure. The phosphoserine signals from the T455A and T455A,S552A samples were less than the phosphoserine signals from the wild type and S552A samples because less radioactivity was applied to the cellulose thin layer plates. The data shown are representative of two independent experiments.
