FIGURE 5. Effect of the T455A mutation on the phosphopeptide map of human CTP synthetase 1 phosphorylated by protein kinase.
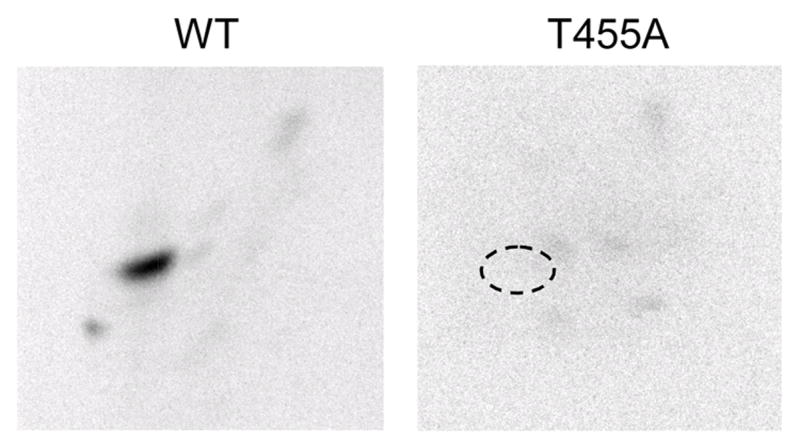
A. The purified wild type (WT) and T455A mutant human CTP synthetase 1 enzymes were phosphorylated with mammalian protein kinase A and [γ-32P]ATP for 10 min. After phosphorylation, the samples were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membrane. The 32P-labeled proteins were digested with L-1-tosylamido-2-phenylethyl chloromethyl ketone-trypsin. The resulting peptides were separated on cellulose thin layer plates by electrophoresis (from left to right) in the first dimension and by chromatography (from bottom to top) in the second dimension. The position of the major phosphopeptide that was absent in the T455A mutant enzyme that was present in the wild type enzyme is indicated in the figure. The data are representative of three independent experiments.
