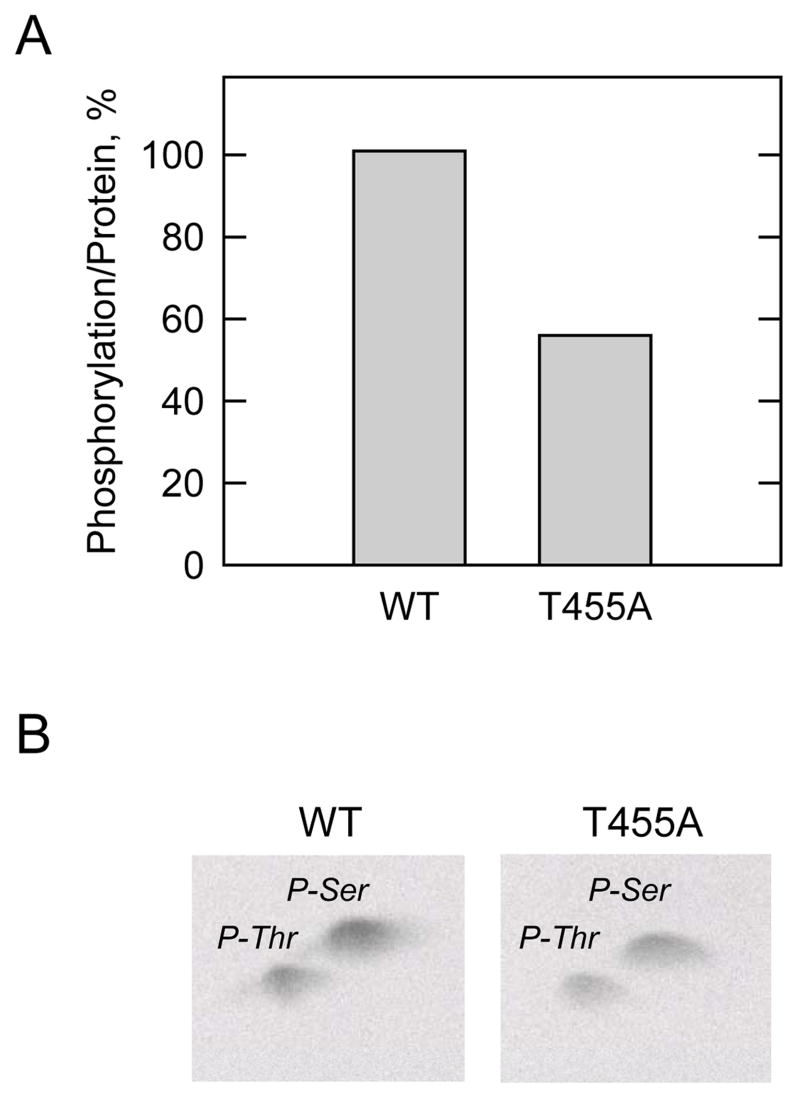
FIGURE 6. Effect of the T455A mutation on the phosphorylation of human CTP synthetase 1 in vivo, and phosphoamino acid analysis of phosphorylated proteins.
A, Cultures (50 ml) of S. cerevisiae ura7Δ ura8Δ cells expressing the wild type (WT) and T455A mutant human CTP synthetase 1 enzymes were grown to the exponential phase of growth. Cells were harvested and resuspended in 5 ml of fresh medium containing 32Pi (0.25 mCi/ml) and incubated for 3 h. Following the incubation, the human CTP synthetase 1 proteins were purified from cell extracts with Ni2+-NTA resin, subjected to SDS-PAGE, and transferred to polyvinylidene difluoride membrane. The 32P-labeled human CTP synthetase 1 proteins were visualized by phosphorimaging analysis, and their relative intensity was quantified using ImageQuant software. The maximum extent of wild type CTP synthetase 1 phosphorylation was set at 100%. The data were normalized to the amount of human CTP synthetase 1 proteins as determined by immunoblot analysis using anti-His6 antibodies. The data are representative of two independent experiments. B, the 32P-labeled wild type and T455A mutant human CTP synthetase 1 enzymes on polyvinylidene difluoride membranes were hydrolyzed with 6N HCl, and the hydrolysates were separated on cellulose thin layer plates by two-dimensional electrophoresis. The positions of the carrier standard phosphoamino acids phosphoserine (P-Ser) and phosphothreonine (P-Thr) are indicated in the figure. The data shown are representative of two independent experiments.