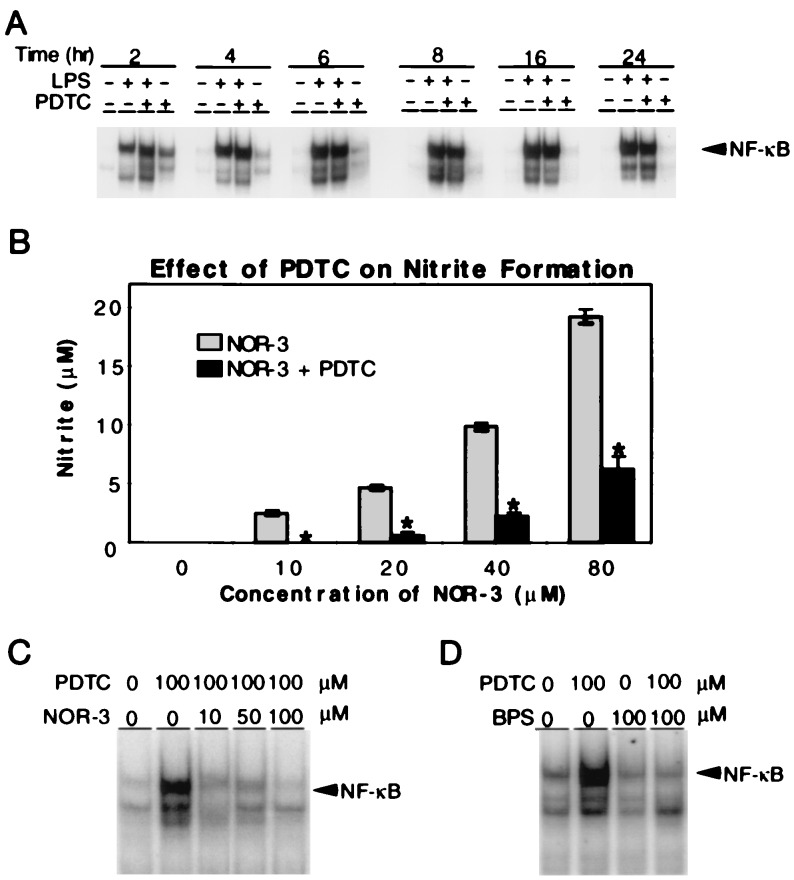
Figure 3.
PDTC enhances NFκB binding in glia by scavenging NO. (A) Electrophoretic mobility shift assays show that LPS (60 ng/ml) potently activates NFκB binding in astrocytes at 2 h, and this activation continues to 24 h. PDTC (100 μM) enhances LPS-mediated NFκB activation for the first 6 h after LPS administration. At 8 to 24 h PDTC modestly inhibits LPS-stimulated NFκB binding. Notably, PDTC (100 μM) alone activates NFκB binding at 2 h with effects diminishing at 6 h of PDTC treatment. (B) PDTC (100 μM) significantly inhibits NOR-3-mediated nitrite formation at all concentrations of NOR-3. Results represent the mean of five independent determinations ± the SD. ∗, P < 0.01 as compared with NOR-3 alone. (C) Electrophoretic mobility shift assays indicate that NOR-3 (10–100 μM) blocks PDTC (100 μM) enhancement of NFκB activity by effectively counteracting the NO-scavenging capabilities of PDTC. (D) Electrophoretic mobility shift assays demonstrate that BPS (100 μM) effectively inhibits PDTC (100 μM) enhancement of NFκB activity in astrocytes. A, C, and D are representative of at least three independent experiments.