Abstract
Aims
To determine the prevalence of isolated tumour cells (ITC) in lymph nodes of patients with pathological node‐negative (pN0) tumours and to assess their impact on disease‐free and overall survival.
Methods
Paraffin embedded lymph nodes from oesophagogastrectomy specimens were examined immunohistochemically using monoclonal anti‐cytokeratin antibody (MNF118). Clinical and pathological features were summarised and overall and relapse‐free survival were estimated.
Results
Isolated tumour cells were detected in 12 of 146 patients (8%), and 24 of 1694 (1%) lymph nodes. With a median follow‐up time of 28 months (range 0–160 months), both relapse‐free and overall survival were significantly (p<0.05) associated with the presence of ITC in pN0 lymph nodes. There was no significant difference in the prevalence of ITC between patients who underwent multimodal therapy and those treated with surgery alone.
Conclusions
ITC in pN0 lymph nodes may be less frequent than previously considered, but their presence is associated with poorer outcomes compared with true node negative disease.
Keywords: esophageal neoplasms, esophagogastric junction, neoplasm metastasis, lymphatic metastasis, immunochemistry
Despite improvements in surgical outcomes and the advent of multimodality regimens, the overall outlook in oesophagogastric cancer remains bleak, with only 8–20% of patients alive at 5 years.1 The presence or absence of metastases to regional lymph nodes is the single most important standard risk factor for patients with oesophagogastric cancers, and even in patients with pT1 tumours the presence of lymph node metastasis has been reported to decrease overall 5‐year survival by up to 100%.2 A large sub‐group of lymph node negative (pN0) patients recur, either locally or systemically, and usually within two years of surgery. It has been hypothesised that early recurrence following complete resection of an apparently localised primary lesion is attributable to disseminated tumour cells which were not detected by routine staging methods at the time of surgery.3 Better methods for detecting and characterising subclinical metastatic deposits in various compartments of the body could enable us to both refine our estimates of the risk of recurrence for individual patients and perhaps tailor therapy more effectively.4 This study evaluates isolated tumour cells in lymph nodes of a large cohort of patients pathologically staged as node negative disease (pN0) and sets out to establish the prevalence and prognostic significance of such cells.
Patients and methods
Patients
All patients with pN0 tumours who underwent curative therapy for oesophagogastric cancer between January 1990 and September 2002 were identified from the prospectively compiled upper gastrointestinal cancer database at this institution. All patients with overt metastatic (M1) disease were excluded and only adenocarcinomas or squamous cell carcinomas were included. The study group consisted of 146 patients: 76 (52%) underwent surgery alone and 70 (48%) underwent a multimodality regimen as previously described.5 This consisted of concurrent chemotherapy (cisplatin and 5‐fluorouracil) and radiotherapy (40 Gy/2 Gy per fraction) followed by oesophagogastrectomy at a median of 4 (range 3–6) weeks following neo‐adjuvant therapy.
Methods
This study was approved by the local hospital ethics committee (St James's Hospital and Federated Dublin Voluntary Hospital joint research ethics committee). Routine pathological staging at this institution is as follows. H&E slides are reviewed in all cases by a consultant pathologist. Resected specimens are fixed in 10% buffered formalin. Where no obvious macroscopic tumour remains, the site of tumour seen at endoscopy is entirely submitted for histological examination. Full thickness transverse sections of the wall are taken.
The mean number of blocks taken for each case was 10 (range 4–17) and the mean number of tumour/ulcer blocks taken for each case was 4 (range 3–10). Perioesophageal and greater and lesser curve lymph nodes were dissected where present and submitted separately for histology. For the purpose of the study an additional (4 μm) section of each resected node was taken off the surface of paraffin embedded lymph node tissue.
Immunostaining was performed as follows: each section was baked at 60°C overnight, deparaffinised and rehydrated through xylenes and graded alcohol series. Endogenous peroxidase activity was blocked by incubating the slides in 3% hydrogen peroxide (Sigma‐Aldrich Ireland Ltd) in water for 10 min. The slides were washed in deionised water for 5 min and digested for 5 min at room temperature with 0.05% proteinase (Sigma‐Aldrich Ireland Ltd) made in 0.005 M Tris‐buffered saline (pH 7.6) (TBS). The slides were then washed in TBS for 5 min. The tissue sections were blocked with 5% bovine serum albumin (Sigma‐Aldrich Ireland Ltd) for 10 min, blotted dry and then incubated with mouse anti‐human cytokeratin (Clone MNF 116, DakoCytomation Ltd, UK) (1:300 in 5% bovine serum albumin) in a humid chamber for 1 hour at room temperature. The slides were washed in TBS for 5 min and incubated with a biotinylated secondary antibody (1:300 in TBS) (rabbit anti‐mouse immunoglobulin, DakoCytomation Ltd, UK) for 30 min and then avidin‐horseradish peroxidase (Vectastain Elite ABC Kit; Vector Laboratories Ltd, UK) for 30 min. The slides were washed with TBS for 5 min after each incubation. The stain was developed by covering each specimen with a 3′3′‐diaminobenzidine solution (DakoCytomation Ltd, UK) for 5 min or until desired stain intensity was achieved. Finally each slide was counterstained with haematoxylin, cleared and mounted.
The immunostained slides were evaluated by an experienced pathologist, who was blinded to patient information, and were scored as positive for isolated tumour cells (ITC) if they contained single or small clusters of strongly immunoreactive epithelial cells in the subcapsular sinus or in the cortex of the lymph node. Patients were pathologically staged as per the 6th AJCC Cancer Staging Manual6 with the addition of TNM shorthand notation for isolated tumour cells first proposed by Hermanek and colleagues.7 Positive cases were designated as pN0(i+) and negative as pN0(i−). With each run, sections of primary tumours were used as positive controls and a negative control (primary antibody omitted) was always included.
Statistical analysis
Statistical calculations were performed using JMP software V.5.1.2 for Macintosh (SAS Institute, Cary, NC, USA). The Kaplan–Meier survival model was used to estimate survival. The log rank and Wilcoxon tests were used to determine statistical differences between groups. Analysis of the predictive value of clinicopathological variables for ITC was performed using the Kruskal–Wallis test for continuous variables and χ2 with Pearson test for categorical data. Cox's proportional hazard model was fitted to multivariate analysis. The following variables were controlled for in the model: gender, age, tumour site, tumour morphology, degree of differentiation, treatment modality and presence or absence of isolated tumour cells. All tests were two sided; results were considered significant at p<0.05.
Results
Table 1 shows demographic and clinical details of the study group. The median age of the patients was 63 (range 29–84) years. There were 101 (69%) men and 45 (41%) women. A total of 1694 lymph nodes were dissected from the 146 oesophagogastrectomy specimens with a mean of 11 nodes per patient. Follow‐up data were obtained for all 146 patients. The median follow‐up time was 28 months (range 0–160 months) and the median actuarial overall survival time was 21 months (95% quartiles: 34–64 months) for the entire study group.
Table 1 Patient demographics.
| Variable | No patients (%) |
|---|---|
| Male | 101 (69%) |
| Female | 45 (31%) |
| Male:female | 2.2:1 |
| Tumour site | |
| Upper oesophagus | 3 (2%) |
| Middle oesophagus | 21 (14%) |
| Lower oesophagus | 57 (39%) |
| OG junction | 65 (45%) |
| Histology | |
| Squamous cell carcinoma | 51 (35%) |
| Adenocarcinoma | 95 (65%) |
| Differentiation | |
| Well | 18 (12%) |
| Moderately | 69 (46%) |
| Poorly | 37 (27%) |
| Undifferentiated | 22 (15%) |
| Pathological tumour stage | |
| Tis | 5 (3%) |
| T0 | 30 (21%) |
| T1 | 29 (20%) |
| T2 | 19 (13%) |
| T3 | 60 (41%) |
| T4 | 3 (2%) |
OG, oesophagogastric; Tis, tumour in situ.
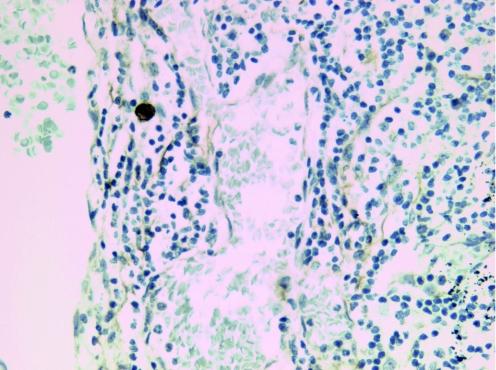
Positive MNF116 staining with malignant morphology was found in 24 of 1694 lymph nodes (1%) and in 12 of 146 patients (8%) studied. ITC was predominantly identified in the subcapsular sinuses either as a single cell (fig 1) or as small clusters of tumour cells.
Figure 1 An example of a single isolated tumour cell (immunohistochemistry).
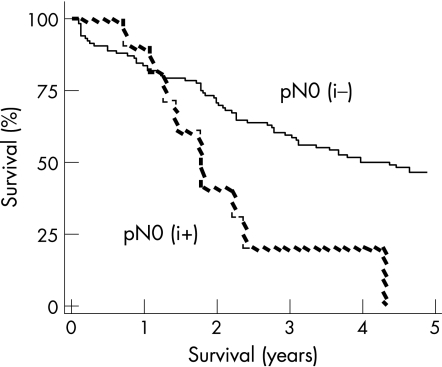
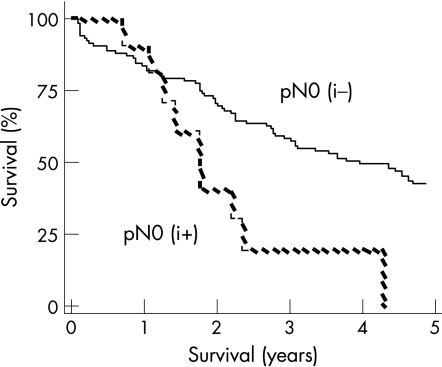
At a median follow‐up of 28 months (range 0–160 months), 59 patients were alive (four with evidence of relapse) and 87 patients had died. The overall and relapse‐free actuarial survival rates were significantly worse among patients who were pN0(i+). The actuarial median overall survival in the pN0(i−) patients was 53 months (95% quartiles: 27–69 months) versus 21 months (9–28) for the pN0(i+) patients (p<0.05) (fig 2). The relapse‐free actuarial survival rate was 40 (27–56) months for the pN0 (i–) patients versus 21 (9–28) months for the pN0 (i+) patients (p<0.05) (fig 3).
Figure 2 Overall survival (Kaplan–Meier survival curve).
Figure 3 Relapse free survival (Kaplan–Meier survival curve).
No significant correlation was found between isolated tumour cells and a number of clinicopathological parameters including age, gender, tumour location, treatment modality, tumour stage, tumour grade, tumour length or mean number of lymph nodes examined. Although not significant, higher TNM staging tended to increase the probability of ITC (table 2). Multivariate analysis revealed that degree of differentiation, tumour morphology and the presence of isolated tumour cells were independent prognostic factors for both relapse‐free and overall survival (table 3).
Table 2 Relationships between various clinicopathological parameters and findings of isolated tumour cells.
| Variable | Positive (%) | Negative (%) | p‐Value |
|---|---|---|---|
| All patients | 12 (8) | 134 (92) | |
| Male | 7 (7) | 94 (93) | NS |
| Female | 5 (11) | 40 (89) | |
| Age | 60 (9) | 61 (10) | NS |
| Mean (SD) | |||
| Pathological tumour stage | |||
| Tis | 0 (0) | 4 (100) | |
| T0 | 1 (4) | 26 (96) | |
| T1 | 1 (4) | 25 (96) | NS |
| T2 | 2 (11) | 17 (89) | |
| T3 | 5 (9) | 49 (91) | |
| T4 | 2 (100) | 0 (0) | |
| Histology | |||
| Squamous cell carcinoma | 1 (2) | 48 (98) | |
| Adenocarcinoma | 11 (12) | 82 (88) | NS |
| Other | 0 (0) | 4 (100) | |
| Differentiation | |||
| Well | 1 (6) | 17 (94) | |
| Moderately | 4 (6) | 65 (94) | NS |
| Poorly | 6 (16) | 31 (83) | |
| Undifferentiated | 1 (5) | 20 (95) | |
| Tumour site | |||
| Upper oesophagus | 0 (0) | 3 (100) | |
| Middle oesophagus | 0 (0) | 21 (100) | NS |
| Lower oesophagus | 5 (9) | 52 (91) | |
| OG junction | 7 (11) | 58 (89) | |
| Treatment | |||
| Surgery alone | 9 (12) | 67 (88) | NS |
| Multi‐modal | 3 (4) | 67 (96) | |
| Barrett's oesophagus | |||
| Yes | 5 (12%) | 37 (88%) | NS |
| No | 7 (7%) | 96 (93%) | |
| No of dissected lymph nodes, mean (SD) | 16 (9) | 11 (13) | NS |
| Tumour length (cm) | 2.7 (0.9) | 3.5 (1.9) | NS |
OG, oesophagogastric; Tis, tumour in situ.
Table 3 Multivariate analysis for prognosis after resection for pathological node‐negative gastro‐oesophageal carcinoma.
| Factor | Risk ratio (95% CI) |
|---|---|
| ITC (yes/no) | 2.92 (1.32 to 5.91) |
| Gender | 0.71 (0.52 to 0.96) |
| Age at diagnosis (⩾63 years, <63 years) | 1.05 (0.81 to 1.08) |
| Tumour site (upper/lower/middle/OG junction) | 0.79 (0.49 to 1.39) |
| Morphology (adenocarcinoma/squamous cell carcinoma) | 0.33 (0.16 to 0.94) |
| Differentiation (well, moderate, poor) | 1.84 (1.28 to 2.69) |
| Treatment (multi‐modal/surgery) | 1.11 (0.88 to 1.41) |
ITC, isolated tumour cells; OG, oesophagogastric.
Discussion
A variety of techniques have been developed to demonstrate occult tumour cells at sites such as the bone marrow, blood and lymph nodes of patients with breast cancer, colon cancer, non‐small‐cell lung cancer, prostate cancer, melanoma and oesophagogastric cancers.8,9,10,11,12 Both in vivo and in vitro studies have suggested that these cells not only have the phenotype of malignant cells, but also possess malignant molecular characteristics.13,14,15,16 Nevertheless the prognostic significance of these cells remains controversial, and a number of studies and a meta‐analysis have failed to verify the presence of occult metastases as an independent prognostic factor in solid tumours.17
One confounding variable in the interpretation of the literature to date has been the inconsistency in nomenclature for “metastases” detected by these methods. They have been inconsistently classified inter alia as micrometastases, subclinical metastases, occult metastases, and tumour cell micro‐involvement. The International Union Against Cancer has tried to clarify the terminology by making a distinction between “micrometastases” and “isolated tumour cells”.7 Micrometastases are defined as being ⩽2 mm in greatest dimension, in contact with a vessel wall, extravasated, proliferating and usually associated with a stromal reaction. Isolated tumour cells (ITC) in contrast are defined as clusters (<0.2 mm) or single tumour cells without any of the above characteristics whose presence can only be determined by immunohistochemistry, immunocytochemistry or molecular methods such as flow cytometry or PCR.
This study to our knowledge is the largest evaluation of the prevalence and prognostic significance of isolated tumour cells in patients reported as pN0 after curative treatment for localised cancer of the oesophagus or oesophagogastric junction. The study reports a low prevalence of isolated tumour cells in pN0 lymph nodes but a significant impact on overall and relapse‐free survival.
We have identified 12 previous studies examining the prevalence and prognostic impact of occult lymph node metastases in oesophagogastric cancer.2,18,19,20,21,22,23,24,25,26,27,28 Seven of these studies reported a significant impact on survival,20,21,22,23,26,27,28 four did not show significance,2,19,24,25 and one paper did not specify prognostic impact with respect to pN0 cases.18 This study differs from these earlier studies in a number of respects. We report on a larger series of pN0 patients than previous studies. Patients with pathologically involved nodes (pN1) were excluded, in contrast to other series.2,18,20,21,22,23,26 With the exception of a study by Vazquez‐Sequeiros et al,25 which reported a prevalence rate of 9%, the rate of 8% in this series was considerably lower than those quoted in previous studies which ranged from 26% to 62%.
There are a number of potential explanations for this low prevalence rate. Many pathologists report as relatively common a finding of single epithelial cells identified by immunohistochemistry for cytokeratin that on careful analysis was found to be hyalinised cytokeratin particles or artefact of overlay of cells from the staining solutions, water bath, or keratinocytes from the skin of the hands of the technician.29 These were also encountered during the course of this study, but only positively staining material with both cellular and malignant morphology as determined by an experienced histopathologist was classified as positive, consistent with current consensus.4
A significant proportion of pN0 patients will be reclassified as pN1 when additional lymph nodes are sampled in a systematic manner,30,31 and it is not unreasonable to infer that the likelihood of finding isolated tumour cells directly correlates with the number of sampled nodes. It is notable that studies such as this from the western world report much smaller nodal yield compared with Japanese series in particular.19,23,24,26,27 Notwithstanding this possibility, we did not find a significant difference in the average numbers of nodes sampled per case between the positive and negative groups in this study.
There is some experimental evidence that the greater the number of sections sampled per lymph node, the higher is the probability of identifying isolated tumour cells or micrometastases.18,32 It is also clear that to serially section and immunostain every resected node after an en bloc oesophagogastrectomy is not a practical proposition for routine pathology laboratories. Accordingly investigators make an empiric decision to sample somewhere between 1 and 5 sections per lymph node. We elected to sample one 4 μm section per lymph node because this was standard practice at this institution and would therefore be a procedure that we could expect to be practicable should this investigation suggest itself to be of clinical relevance.
Take‐home messages
The prevalence of isolated tumour cells in pathological node negative (pN0) lymph nodes of oesophagogastric cancer may be lower than previously reported.
Studying a larger cohort than any previously reported and using stringent detection criteria, it is reported that the presence of isolated tumour cells in pN0 oesophagogastric cancer lymph nodes has prognostic significance.
In summary, this study shows that the prevalence of isolated tumour cells in pN0 lymph nodes of oesophagogastric cancer may be lower than previously reported, and that the presence of isolated tumour cells has prognostic significance when analysed in a sufficiently large cohort using stringent detection criteria. The integration of ITC detection into the routine staging of oesophagogastric cancers could improve our ability to determine prognosis and bring the realisation of the goal of patient‐tailored cancer therapy closer.
Abbreviations
ITC - isolated tumour cells
pN0 - pathological node‐negative
Footnotes
Competing interests: None declared.
References
- 1.Enzinger P C, Mayer R J. Esophageal cancer. N Engl J Med 20033492241–2252. [DOI] [PubMed] [Google Scholar]
- 2.Glickman J N, Torres C, Wang H H.et al The prognostic significance of lymph node micrometastasis in patients with esophageal carcinoma. Cancer 199985769–778. [PubMed] [Google Scholar]
- 3.Pantel K, Izbicki J, Passlick B.et al Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non‐small‐cell lung cancer without overt metastases. Lancet 1996347649–653. [DOI] [PubMed] [Google Scholar]
- 4.Lugo T G, Braun S, Cote R J.et al Detection and measurement of occult disease for the prognosis of solid tumors. J Clin Oncol 2003212609–2615. [DOI] [PubMed] [Google Scholar]
- 5.Walsh T N, Noonan N, Hollywood D.et al A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996335462–467. [DOI] [PubMed] [Google Scholar]
- 6.Greene F L, Page D L, Fleming I D.et alAJCC cancer staging manual, 6th edn. New York, NY: Springer‐Verlag 2002
- 7.Hermanek P, Hutter R V, Sobin L H.et al International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer 1999862668–2673. [PubMed] [Google Scholar]
- 8.Cote R J, Hawes D, Chaiwun B.et al Detection of occult metastases in lung carcinomas: progress and implications for lung cancer staging. J Surg Oncol 199869265–274. [DOI] [PubMed] [Google Scholar]
- 9.Cote R J, Peterson H F, Chaiwun B.et al Role of immunohistochemical detection of lymph‐node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet 1999354896–900. [DOI] [PubMed] [Google Scholar]
- 10.Liefers G J, Cleton‐Jansen A M, van de Velde C J.et al Micrometastases and survival in stage II colorectal cancer. N Engl J Med 1998339223–228. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein R A, Zietman A L, de las Morenas A.et al Implications of prostate micrometastases in pelvic lymph nodes: an archival tissue study. Urology 199647370–375. [DOI] [PubMed] [Google Scholar]
- 12.Cochran A J, Wen D R, Morton D L. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 198812612–618. [DOI] [PubMed] [Google Scholar]
- 13.Muller P, Weckermann D, Riethmuller G.et al Detection of genetic alterations in micrometastatic cells in bone marrow of cancer patients by fluorescence in situ hybridization. Cancer Genet Cytogenet 1996888–16. [DOI] [PubMed] [Google Scholar]
- 14.Klein C A, Schmidt‐Kittler O, Schardt J A.et al Comparative genomic hybridization, loss of heterozygosity, and DNA sequence analysis of single cells. Proc Natl Acad Sci USA 1999964494–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantel K, Dickmanns A, Zippelius A.et al Establishment of micrometastatic carcinoma cell lines: a novel source of tumor cell vaccines. J Natl Cancer Inst 1995871162–1168. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan G C, Sheehan D, Clarke A.et al Micrometastases in esophagogastric cancer: high detection rate in resected rib segments. Gastroenterology 1999116543–548. [DOI] [PubMed] [Google Scholar]
- 17.Funke I, Schraut W. Meta‐analyses of studies on bone marrow micrometastases: an independent prognostic impact remains to be substantiated. J Clin Oncol 199816557–566. [DOI] [PubMed] [Google Scholar]
- 18.Bonavina L, Ferrero S, Midolo V.et al Lymph node micrometastases in patients with adenocarcinoma of the esophagogastric junction. J Gastrointest Surg 19993468–476. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Ide H, Eguchi R.et al Clinical implications of lymph node micrometastasis in patients with histologically node‐negative (pN0) esophageal carcinoma. J Surg Oncol 200279224–229. [DOI] [PubMed] [Google Scholar]
- 20.Mueller J D, Stein H J, Oyang T.et al Frequency and clinical impact of lymph node micrometastasis and tumor cell microinvolvement in patients with adenocarcinoma of the esophagogastric junction. Cancer 2000891874–1882. [DOI] [PubMed] [Google Scholar]
- 21.Izbicki J R, Hosch S B, Pichlmeier U.et al Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med 19973371188–1194. [DOI] [PubMed] [Google Scholar]
- 22.Hosch S B, Stoecklein N H, Pichlmeier U.et al Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001191970–1975. [DOI] [PubMed] [Google Scholar]
- 23.Natsugoe S, Mueller J, Stein H J.et al Micrometastasis and tumor cell microinvolvement of lymph nodes from esophageal squamous cell carcinoma: frequency, associated tumor characteristics, and impact on prognosis. Cancer 199883858–866. [PubMed] [Google Scholar]
- 24.Sato F, Shimada Y, Li Z.et al Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg 200188426–432. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez‐Sequeiros E, Wang L, Burgart L.et al Occult lymph node metastases as a predictor of tumor relapse in patients with node‐negative esophageal carcinoma. Gastroenterology 20021221815–1821. [DOI] [PubMed] [Google Scholar]
- 26.Komukai S, Nishimaki T, Suzuki T.et al Significance of immunohistochemical nodal micrometastasis as a prognostic indicator in potentially curable oesophageal carcinoma. Br J Surg 200289213–219. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Natsugoe S, Nakashima S.et al Clinical significance of lymph node micrometastasis of pN0 esophageal squamous cell carcinoma. Cancer Lett 2000153189–197. [DOI] [PubMed] [Google Scholar]
- 28.Heeren P A M, Kelder W, Blondeel I.et al Prognostic value of nodal micrometastases in patients with cancer of the gastro‐oesophageal junction. Eur J Surg Oncol 200531270–276. [DOI] [PubMed] [Google Scholar]
- 29.Page D L, Anderson T J, Carter B A. Minimal solid tumor involvement of regional and distant sites: when is a metastasis not a metastasis? Cancer 1999862589–2592. [PubMed] [Google Scholar]
- 30.Association of Directors for Anatomic and Surgical Pathology Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol 2001115799–801. [DOI] [PubMed] [Google Scholar]
- 31.Lerut T, Coosemans W, De Leyn P.et al Reflections on three field lymphadenectomy in carcinoma of the esophagus and gastroesophageal junction. Hepatogastroenterology 199946717–725. [PubMed] [Google Scholar]
- 32.Ishida K, Katsuyama T, Sugiyama A.et al Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer 1997791069–1076. [DOI] [PubMed] [Google Scholar]