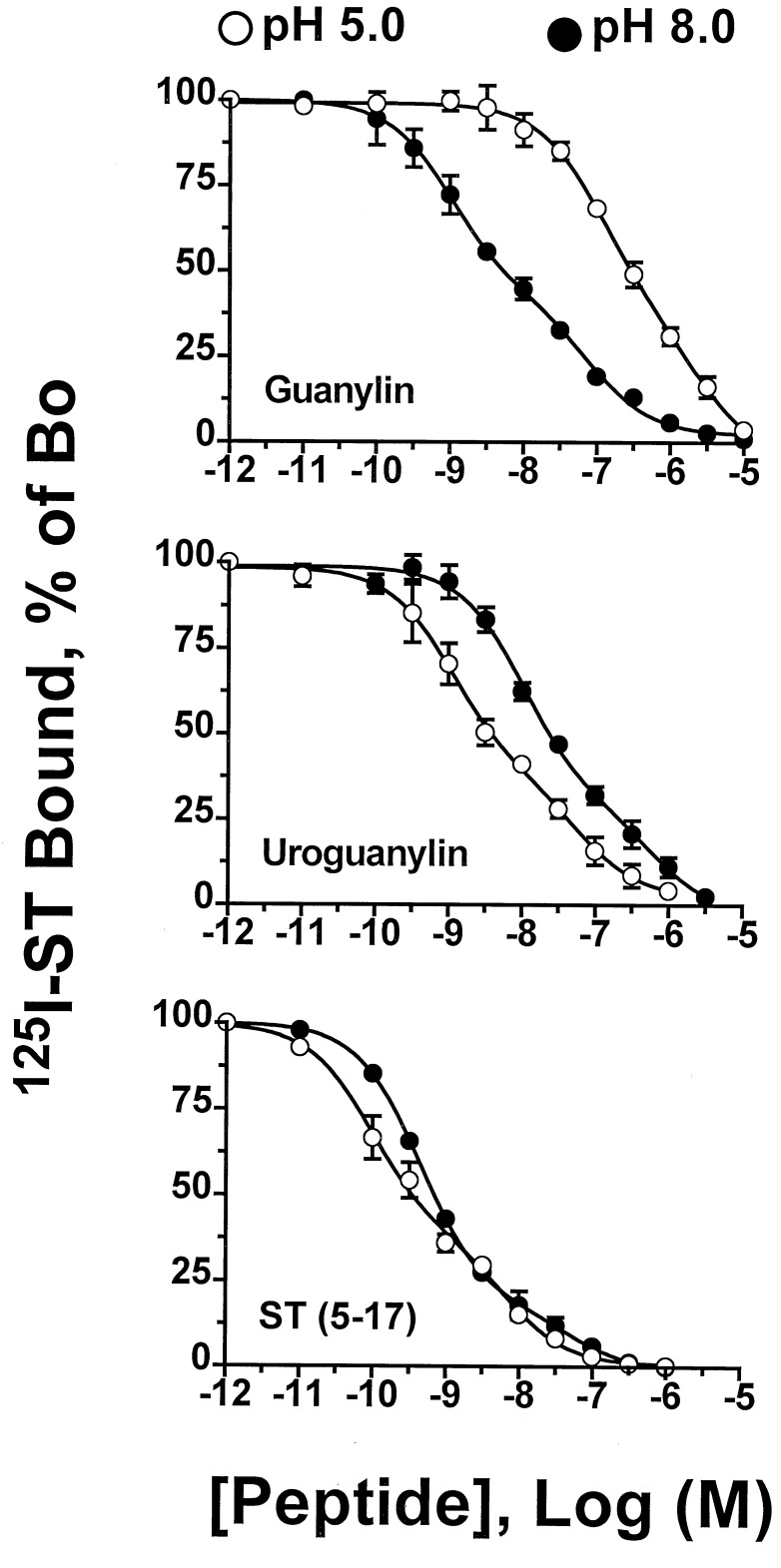
Figure 4.
Effects of medium pH on the relative affinities of guanylin, uroguanylin, and E. coli ST-(5–17) for binding to receptors on T84 cells. Binding of 125I-ST-(1–19) to intact T84 cells was determined in the presence of the indicated concentrations of guanylin (Top), uroguanylin (Middle), and ST-(5–17) (Bottom) as described. The values shown are the composite data (mean ±SEM) from three experiments performed in duplicate with each peptide at pH 5 and pH 8 and are expressed as the total binding of 125I-ST-(1–19) in the absence of a competing ligand. Nonspecific binding was measured using 1 μM ST-(5–17). Competitive radioligand binding curves are computer-derived best fits of the binding data to a two-site model (15). Ki values obtained for the high and low affinity sites were: guanylin, pH 5 ≈102 nM and 2.3 μM, pH 8 ≈1 nM and 77 nM; uroguanylin, pH 5 ≈1 nM and 70 nM, pH 8 ≈10 nM and 615 nM; ST-(5–17), pH 5 ≈94 pM and 7 nM, pH 8 ≈440 pM and 17 nM.