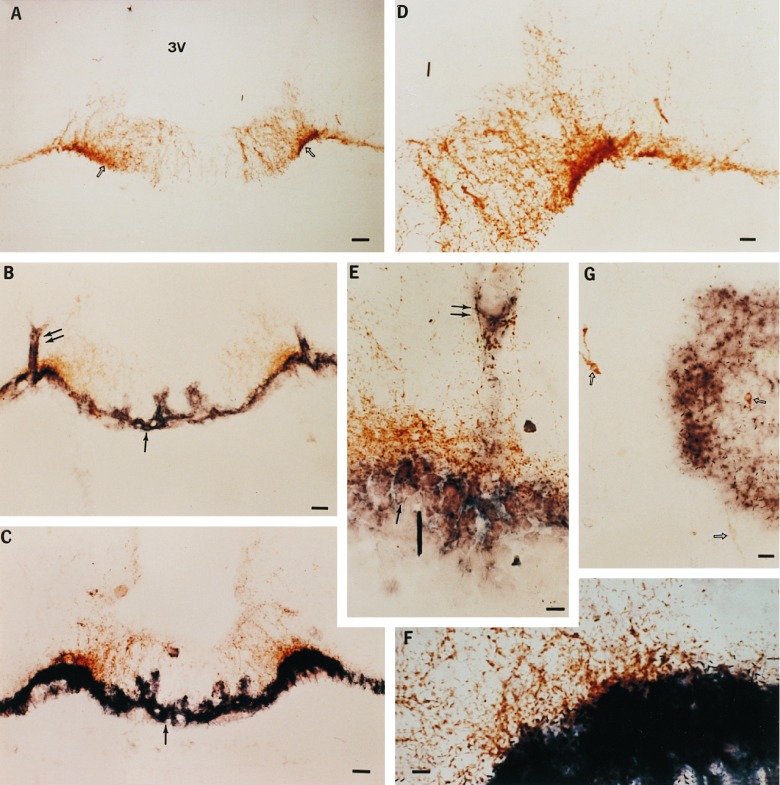
Figure 5.
Localization of TGFα-producing cells transplanted into the hypothalamus of immature female rats by combined immunohistochemistry/in situ hybridization. (A) Low-magnification view (×77) of LHRH nerve terminals (open arrows) in the ME of an animal that received a transplant of control 3T3 cells. 3V, third ventricle. (B) Low-magnification view (×77) of the ME of an animal that underwent precocious puberty after receiving a transplant of TGFα-producing 3T3 cells. In this case TGFα production is continuous, as the TGFα gene inserted into the PGKIRES retrovirus is driven by the constitutively activated PGK promoter. The double arrows denote the sites of microinjection, and the single arrow points to the transplanted cells identified by their content of hTGFα. hTGFα mRNA was detected by hybridization to a digoxigenin-UTP-labeled complementary RNA probe. (C) Low-magnification view (×77) of the ME of an animal that underwent precocious puberty after receiving a transplant of 3T3 cells bearing the hTGFα gene under the control of mouse MT1 inducible promoter. The animal was perfused 5 h after receiving a single subcutaneous injection of zinc chloride to activate the MT promoter. (D, E, and F) Higher-magnification views of A, B, and C, respectively. (D, ×140; E and F, ×420.) (G) View (×140) of the POA of an animal that showed advanced puberty after receiving a transplant of 3T3 cells bearing the PGKIRES-hTGFα retroviral construct into the POA. Notice that the hTGFα-positive cells are in close proximity to a group of LHRH neurons (arrows). (Bars: A, B, and C = 50 μm; D and G = 25 μm; E and F = 10 μm.)