Abstract
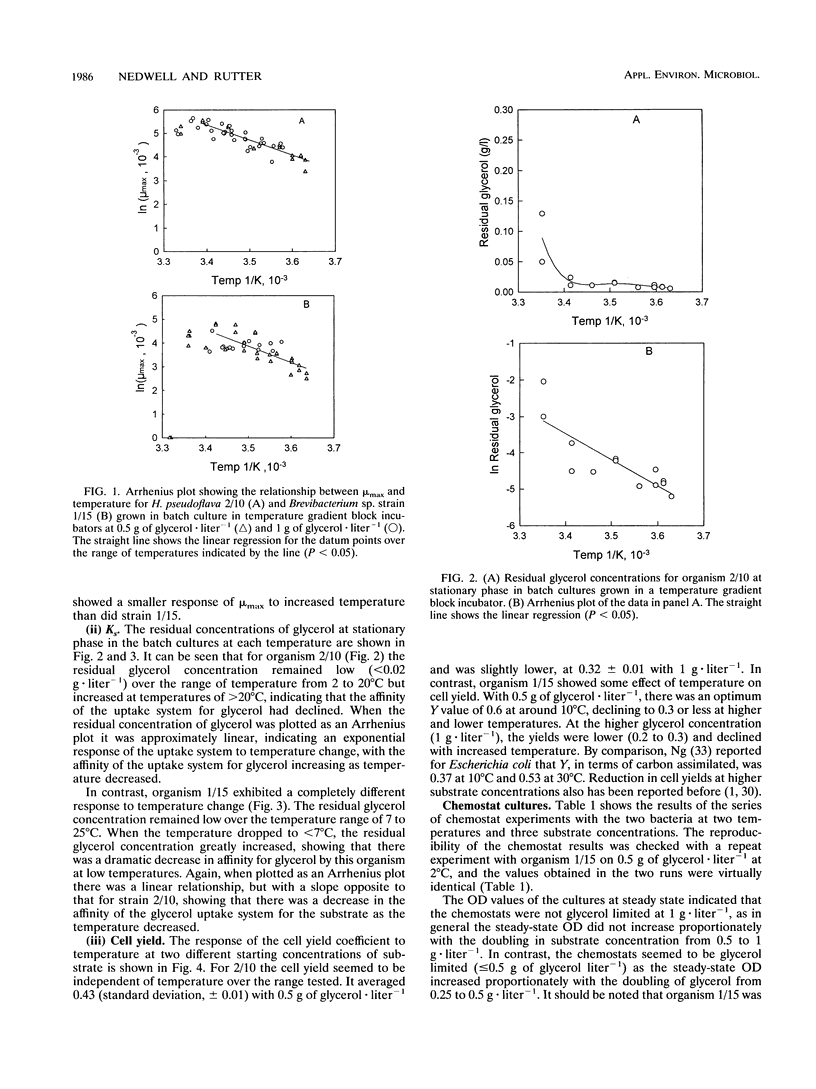
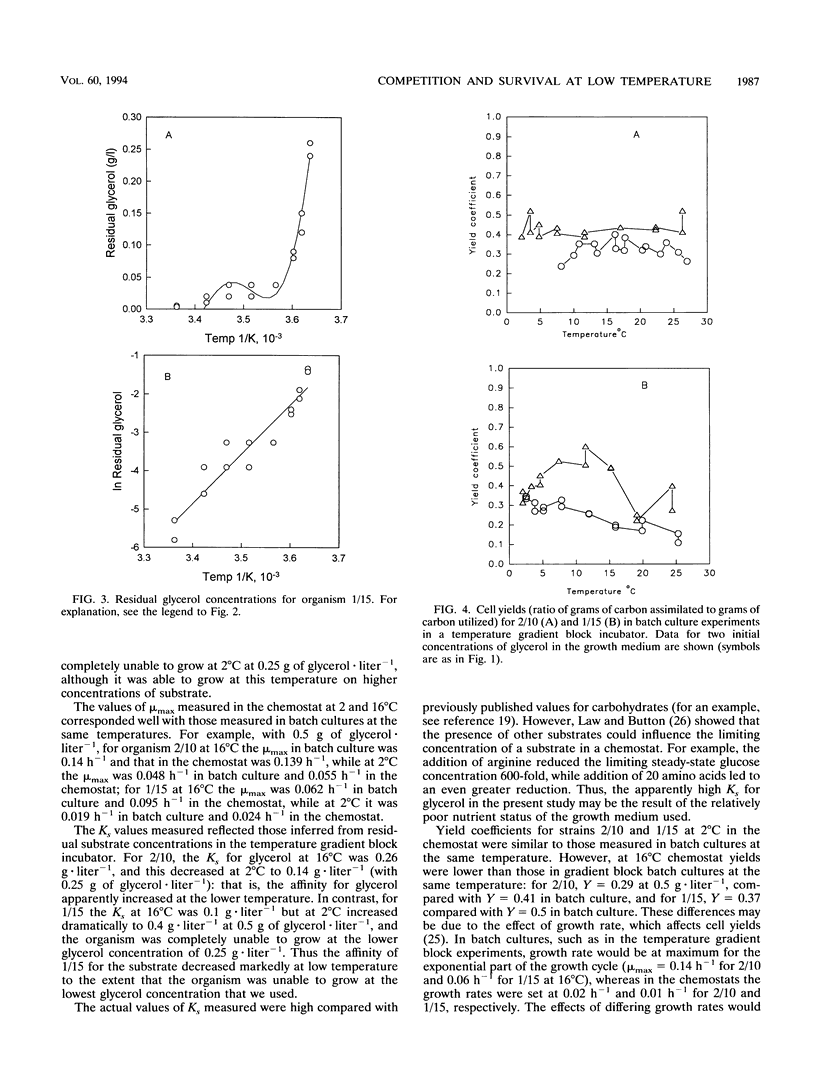
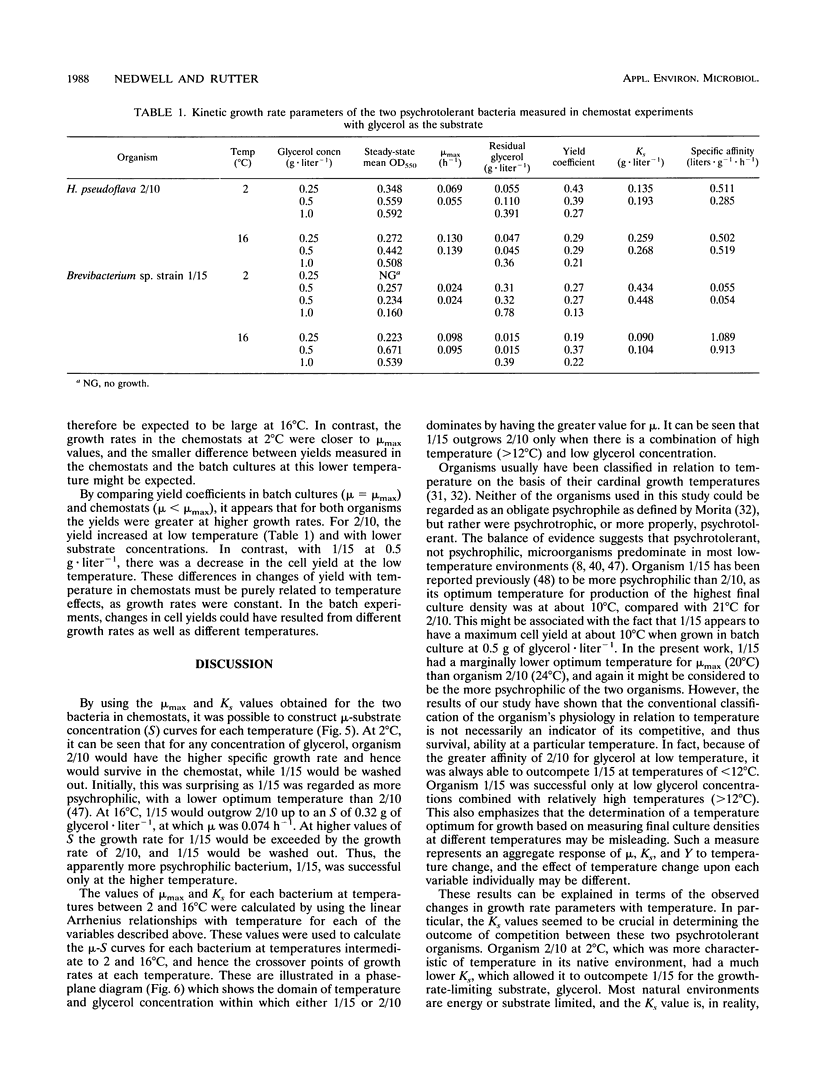
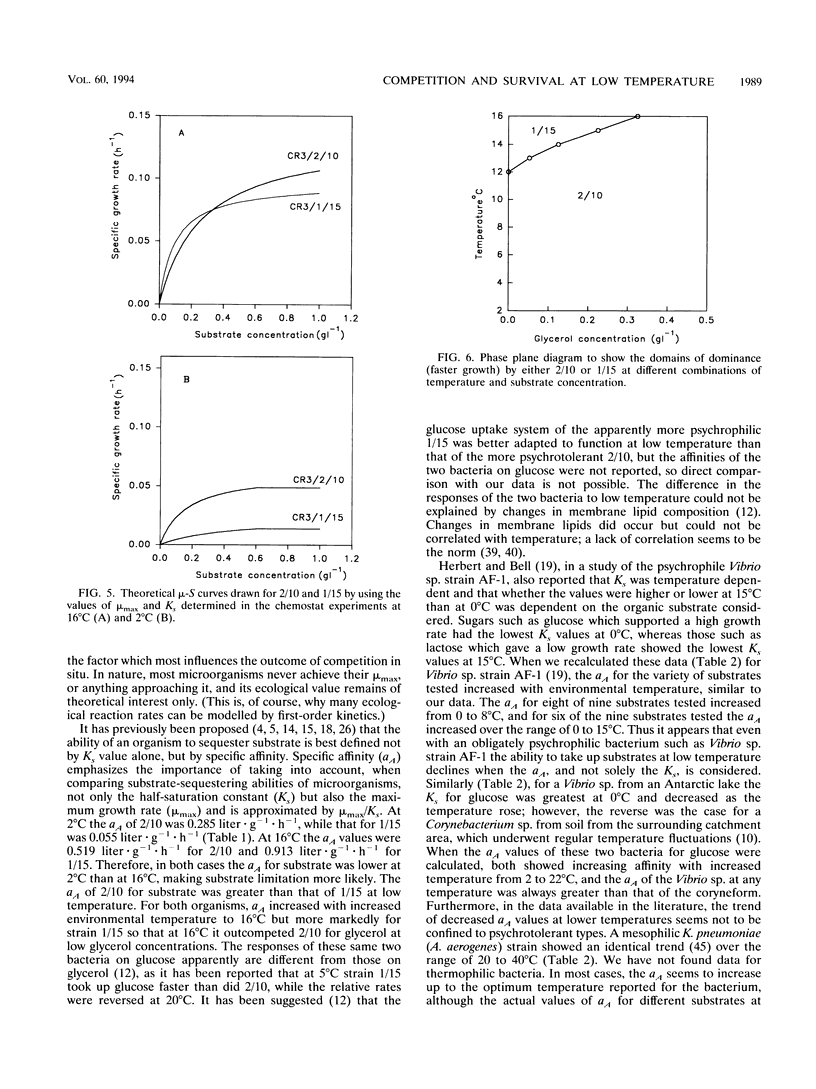
The growth kinetics of two psychrotolerant Antarctic bacteria, Hydrogenophaga pseudoflava CR3/2/10 (2/10) and Brevibacterium sp. strain CR3/1/15 (1/15), were examined over a range of temperatures in both batch culture and glycerol-limited chemostat cultures. The maximum specific growth rate (mu max) and Ks values for both bacteria were functions of temperature, although the cell yields were relatively constant with respect to temperature. The mu max values of both strains increased up to an optimum temperature, 24 degrees C for 2/10 and 20 degrees C for 1/15. Strain 1/15 might therefore be considered to be more psychrophilic than strain 2/10. For both bacteria, the specific affinity (mu max/Ks) for glycerol uptake was lower at 2 than at 16 degrees C, indicating a greater tendency to substrate limitation at low temperature. As the temperature increased from 2 to 16 degrees C, the specific affinity of 1/15 for glycerol increased more rapidly than it did for 2/10. Thus 1/15, on the basis of this criterion, was less psychrophilic than was 2/10. The steady-state growth kinetics of the two strains at 2 and 16 degrees C imply that 1/15 would be able to outgrow 2/10 only at relatively low substrate concentrations (< 0.32 g of glycerol.liter-1) and high temperatures (> 12 degrees C), which suggests that 1/15 has a less psychrotolerant survival strategy than does 2/10. Our data were compared with other data in the literature for bacteria growing at low temperatures. They also showed an increase of substrate-specific affinity with increasing temperature.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Button D. K. Nutrient-limited microbial growth kinetics: overview and recent advances. Antonie Van Leeuwenhoek. 1993;63(3-4):225–235. doi: 10.1007/BF00871220. [DOI] [PubMed] [Google Scholar]
- Ferroni G. D., Kaminski J. S. Psychrophiles, psychrotrophs, and mesophiles in an environment which experiences seasonal temperature fluctuations. Can J Microbiol. 1980 Oct;26(10):1184–1190. doi: 10.1139/m80-198. [DOI] [PubMed] [Google Scholar]
- Gottschal J. C. Some reflections on microbial competitiveness among heterotrophic bacteria. Antonie Van Leeuwenhoek. 1985;51(5-6):473–494. doi: 10.1007/BF00404494. [DOI] [PubMed] [Google Scholar]
- Harder W., Veldkamp H. Competition of marine psychrophilic bacteria at low temperatures. Antonie Van Leeuwenhoek. 1971;37(1):51–63. doi: 10.1007/BF02218466. [DOI] [PubMed] [Google Scholar]
- Herbert R. A., Bell C. R. Growth characteristics of an obligately psychrophilic Vibrio sp. Arch Microbiol. 1977 Jun 20;113(3):215–220. doi: 10.1007/BF00492028. [DOI] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Macleod R. A. Observations on the distinction between oligotrophic and eutrophic marine bacteria. Appl Environ Microbiol. 1984 May;47(5):1017–1022. doi: 10.1128/aem.47.5.1017-1022.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R. Y. Psychrophilic bacteria. Bacteriol Rev. 1975 Jun;39(2):144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H. Effect of decreasing growth temperature on cell yield of Escherichia coli. J Bacteriol. 1969 Apr;98(1):232–237. doi: 10.1128/jb.98.1.232-237.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell N. J. Cold adaptation of microorganisms. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1237):595-608, discussion 608-11. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Rutter M., Nedwell D. B. Influence of changing temperature on growth rate and competition between two psychrotolerant Antarctic bacteria: competition and survival in non-steady-state temperature environments. Appl Environ Microbiol. 1994 Jun;60(6):1993–2002. doi: 10.1128/aem.60.6.1993-2002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala H., Sinclair C. G. Temperature relationship in continous culture. Biotechnol Bioeng. 1971 Nov;13(6):795–813. doi: 10.1002/bit.260130606. [DOI] [PubMed] [Google Scholar]
- Wiebe W. J., Sheldon W. M., Pomeroy L. R. Bacterial growth in the cold: evidence for an enhanced substrate requirement. Appl Environ Microbiol. 1992 Jan;58(1):359–364. doi: 10.1128/aem.58.1.359-364.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


