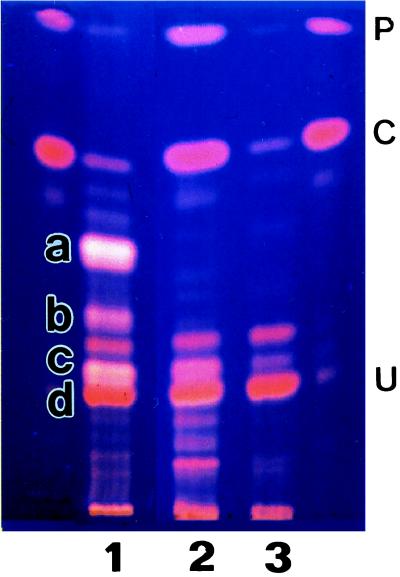
Figure 5.
TLC analysis of methyl esters of porphyrins derived from reaction mixtures containing fractions I and II (lane 1) or fractions I–III (lanes 2 and 3) as enzyme solutions. Fractions I, II, and III were prepared from the soluble fraction of D. vulgaris by DEAE-Toyopearl chromatography (see Fig. 4). Porphyrin esters were detected by their fluorescence with excitation at 366 nm using a Mineralight UVGL-58 (Upland, CA). Standard porphyrin esters: P, protoporphyrin IX dimethyl ester; C, coproporphyrin III tetramethyl ester; U, uroporphyrin III octamethyl ester. Lane 1, experiment 2; a, 12,18-didecarboxysirohydrochlorin hexamethyl ester; b, 12/18-monodecarboxysirohydrochlorin heptamethyl ester; c, sirohydrochlorin octamethyl ester; d, uroporphyrin III octamethyl ester. Lane 2, experiment 3; lane 3, experiment 3 without SAM.