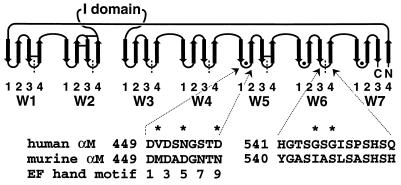
Figure 1.
Topology of the β-propeller model for the N-terminal half of the integrin α-subunit. Each β-sheet (W) contains four anti-parallel β-strands. The Ws are packed into a toroid and around a pseudosymmetry axis in a central cavity that is lined with strand 1 of each W. The FG-GAP repeats, demarcated by vertical dashed lines, are staggered with respect to the Ws. Putative calcium binding loops contain filled circles, and predicted disulfide bonds of Mac-1 are shown by horizontal bars. The I domain is inserted in the loop that connects W2 and W3. The human and mouse Mac-1 amino acid sequences of loop 1–2 of W5 and loop 3–4 of W6 are shown. Residues that contribute to the epitope recognized by mAb CBRM1/20 are indicated with an asterisk. Residues that ligate Ca2+ by sidechains (1, 3, 5, 9) or backbone carbonyl O (7) in EF hands are numbered.