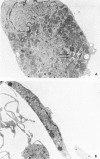
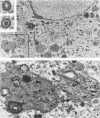
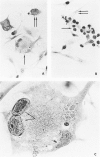
Abstract
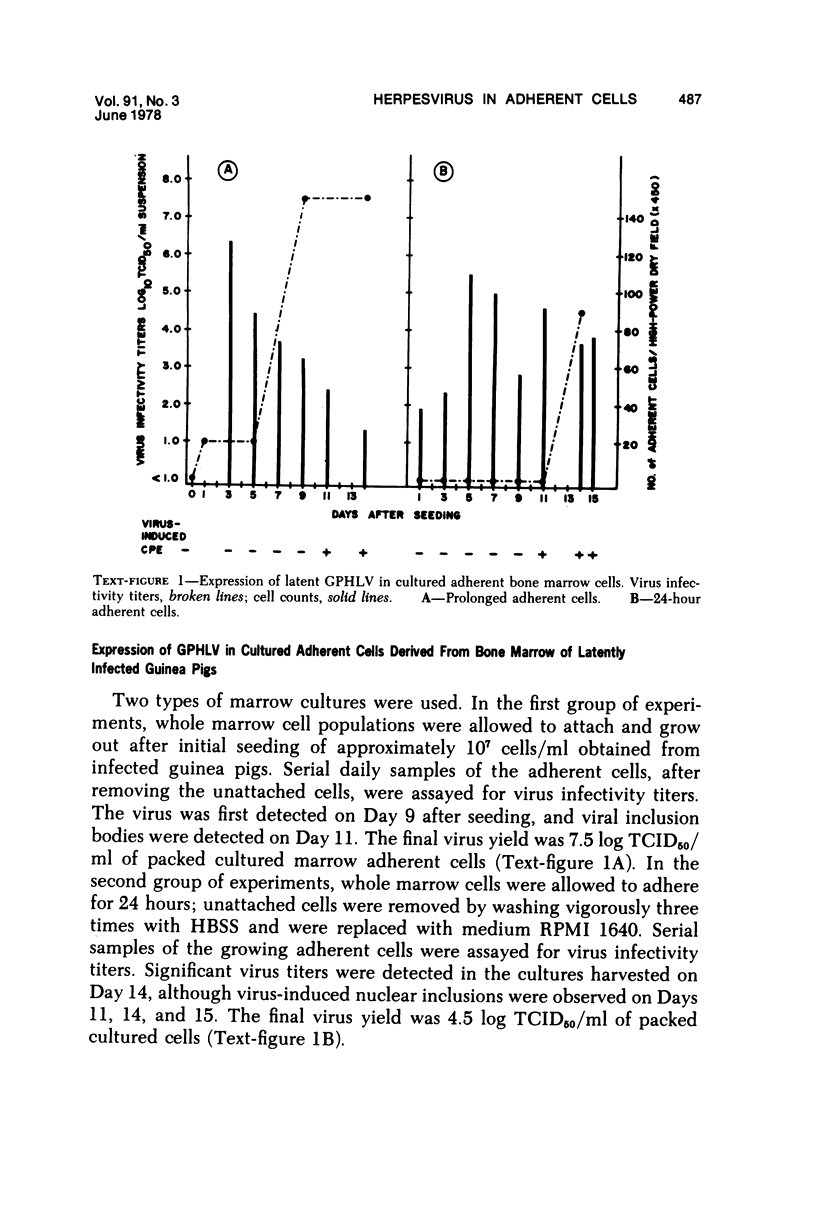
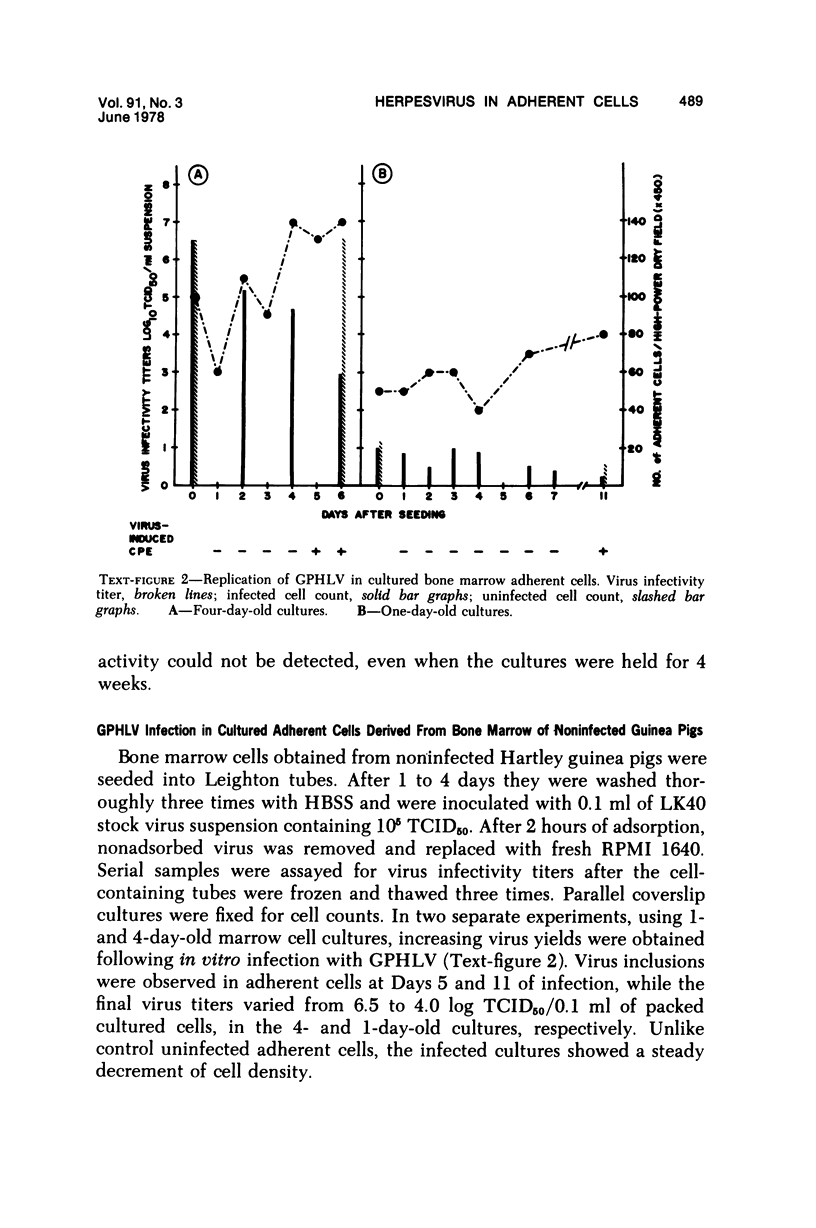
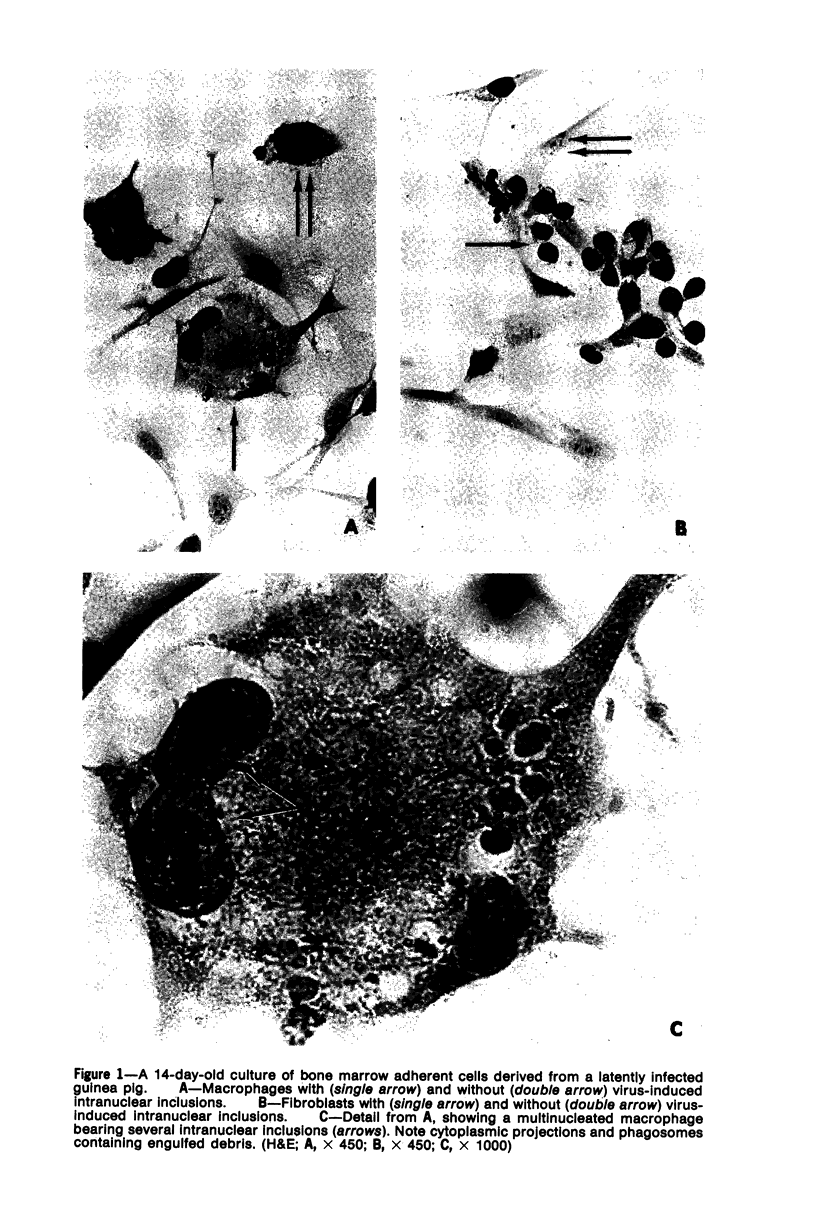
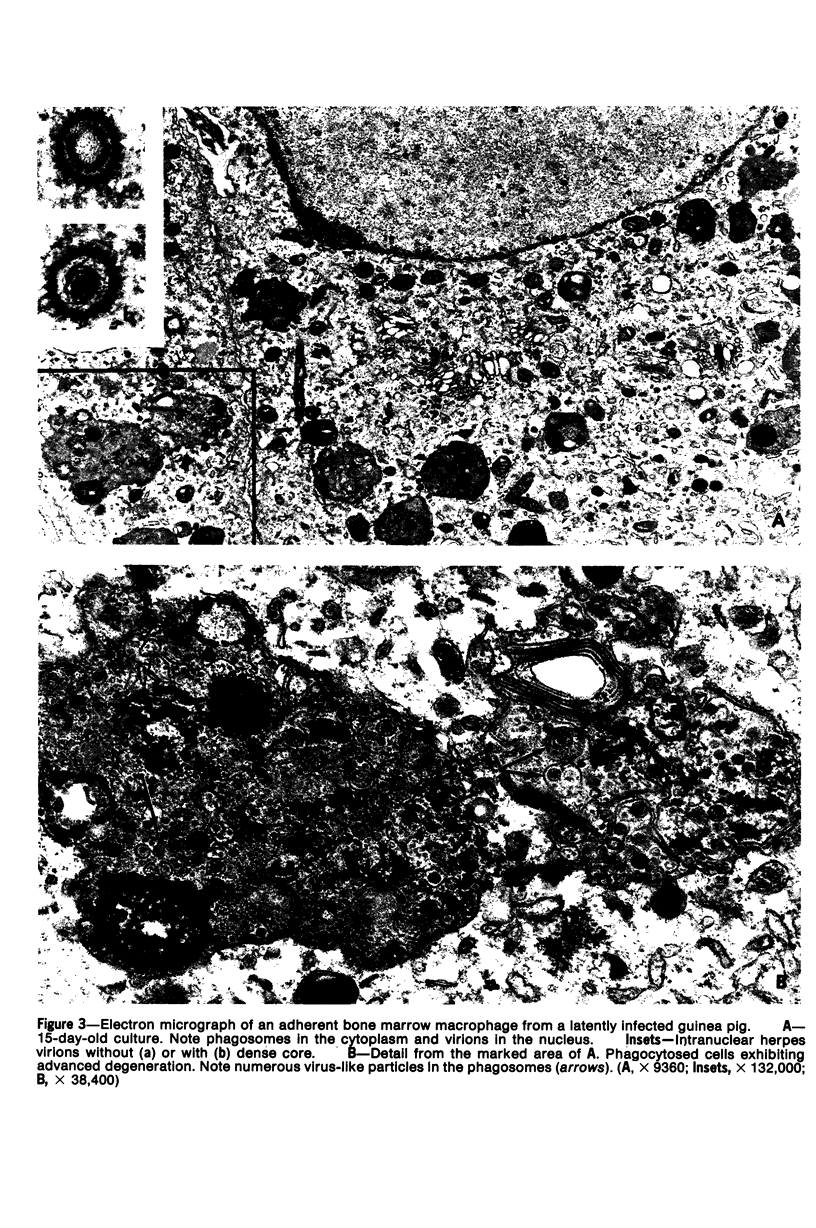
The role of bone marrow adherent cells in the latency of guinea pig herpes-like virus (GPHLV) was explored. Cultures of macrophage-enriched adherent cells derived from infected guinea pigs were examined for evidence of latent GPHLV infection. Expression of the virus was detected in these cultures 9 to 10 days after in vitro cultivation. Increasing virus infectivity titers as well as light and electron microscopic evidence of virion assembly in macrophages and fibroblasts were demonstrated. Infections virus was detected in the bone marrow adherent cells that had attached for 30 or 120 minutes but only following reverse cocultivation. The data showed not only that the bone marrow adherent cells were susceptible to GPHLV in vitro but also that GPHLV was harbored by the macrophage-enriched bone marrow population in vivo in latently infected guinea pigs.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Immune responses in persistent virus infections. J Clin Pathol Suppl (R Coll Pathol) 1972;6:121–131. [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. On the role of mononuclear phagocytes in immunity against viruses. Prog Med Virol. 1974;18(0):15–31. [PubMed] [Google Scholar]
- Booss J., Hsiung G. D. Herpes-like virus of the guinea pig: propagation in brain tissue of guinea pigs and mice. J Infect Dis. 1971 Mar;123(3):284–291. doi: 10.1093/infdis/123.3.284. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Hsiung G. D., Kaplow L. S., Booss J. Herpesvirus infection of guinea pigs. I. Isolation, characterization and pathogenicity. Am J Epidemiol. 1971 Apr;93(4):298–307. doi: 10.1093/oxfordjournals.aje.a121261. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Kaplow L. S. Herpeslike virus isolated from spontaneously degenerated tissue culture derived from leukemia-susceptible guinea pigs. J Virol. 1969 Mar;3(3):355–357. doi: 10.1128/jvi.3.3.355-357.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D., McTighe A. H. An animal model for DNA-RNA virus interaction based upon virological and histological findings. Cancer Res. 1976 Feb;36(2 Pt 2):674–677. [PubMed] [Google Scholar]
- Hsiung G. D., Tenser R. B., Fong C. K. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: growth characteristics and antigentic relationship. Infect Immun. 1976 Mar;13(3):926–933. doi: 10.1128/iai.13.3.926-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller C. A., King G. W., Hurtubise P. E., Sagone A. L., LoBuglio A. F. Characterization of glass adherent human mononuclear cells. J Immunol. 1973 Nov;111(5):1610–1612. [PubMed] [Google Scholar]
- Krishnan A. D., Talwar G. P. Cultivation of human peripheral blood monocytes in vitro. Indian J Med Res. 1974 Mar;62(3):313–316. [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard A., Viken K. E., Lamvik J. Structural and functional properties of blood monocytes cultured in vitro. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):223–234. doi: 10.1111/j.1699-0463.1974.tb02316.x. [DOI] [PubMed] [Google Scholar]
- Shoham D., David E. B., Rozenszajn L. A. Cytochemical and morphologic identification of macrophages and eosinophils in tissue cultures of normal human bone marrow. Blood. 1974 Aug;44(2):221–233. [PubMed] [Google Scholar]
- Sommerville R. G. Rapid diagnosis of viral infections by immunofluorescent staining of viral antigens in leucocytes and macrophages. Prog Med Virol. 1968;10:398–414. [PubMed] [Google Scholar]
- St Jeor S., Weisser A. Persistence of cytomegalovirus in human lymphoblasts and peripheral leukocyte cultures. Infect Immun. 1977 Feb;15(2):402–409. doi: 10.1128/iai.15.2.402-409.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Infection of thymus cells in vivo and in vitro with a guinea pig herpes-like virus and the effect of antibody on virus replication in organ culture. J Immunol. 1973 Feb;110(2):552–560. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]