Abstract
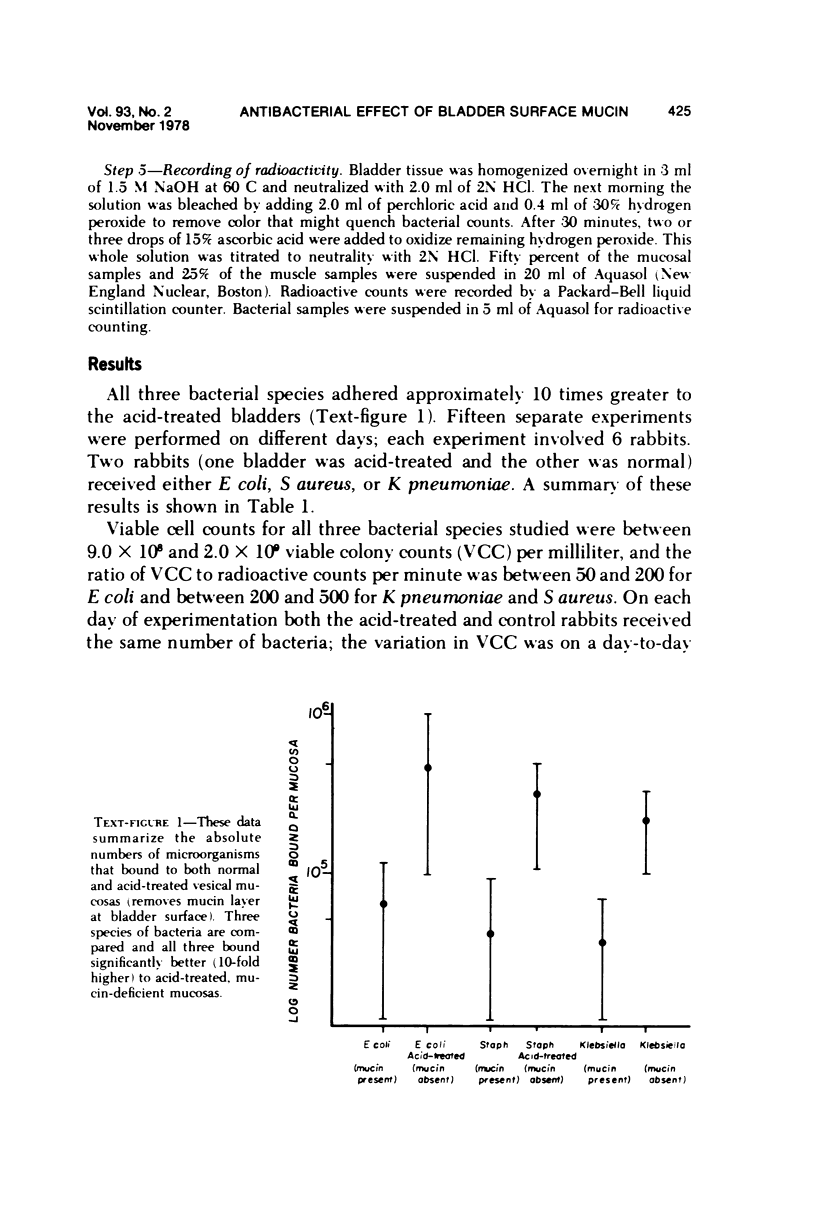
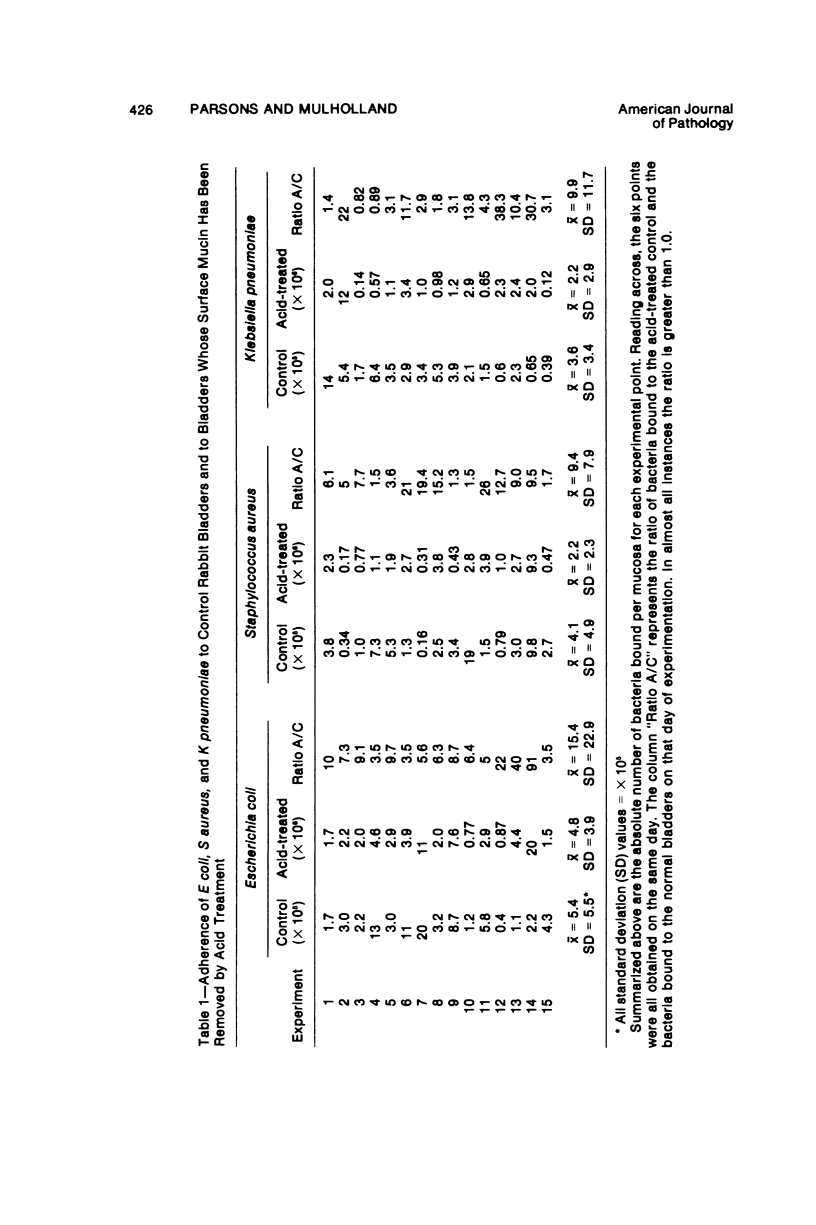
We previously reported the results of quantitative and histochemical studies implicating the surface mucin of the bladder mucosa as an important antibacterial defense mechanism, which functions by preventing bacteria from adhering to the bladder wall. We call the mucin "anti-adherence factor" and we feel this is a previously undocumented role for mucin as a type of host antibacterial defense. These experiments were conduced with Escherichia coli. In an effort to determine whether the anti-adherence ability of the vesical mucin was a generalized phenomenon, we repeated these studies using unrelated bacterial species, including E coli, Klebsiella pneumoniae, and Staphylococcus aureus. The ability of the vesical mucosa to resist bacterial adherence to its surface was found to be independent of the bacterial species that was investigated.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M., Glynn A. A., Lindsay M., Milne C. M. Serological properties of gamma-A antibodies to Escherichia coli present in human colostrum. Immunology. 1966 Jun;10(6):517–526. [PMC free article] [PubMed] [Google Scholar]
- Arbuckle J. B. The location of Escherichia coli in the pig intestine. J Med Microbiol. 1970 May;3(2):333–340. doi: 10.1099/00222615-3-2-333. [DOI] [PubMed] [Google Scholar]
- BOEN J. R., SYLWESTER D. L. THE MATHEMATICAL RELATIONSHIP AMONG URINARY FREQUENCY, RESIDUAL URINE, AND BACTERIAL GROWTH IN BLADDER INFECTIONS. Invest Urol. 1965 Mar;2:468–473. [PubMed] [Google Scholar]
- Bienenstock J., Tomasi T. B., Jr Secretory gamma-A in normal urine. J Clin Invest. 1968 May;47(5):1162–1171. doi: 10.1172/JCI105805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX C. E., HINMAN F., Jr Experiments with induced bacteriuria, vesical emptying and bacterial growth on the mechanism of bladder defense to infection. J Urol. 1961 Dec;86:739–748. doi: 10.1016/S0022-5347(17)65257-1. [DOI] [PubMed] [Google Scholar]
- Cobbs C. G., Kaye D. Antibacterial mechanisms in the urinary bladder. Yale J Biol Med. 1967 Oct;40(2):93–108. [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker M. M., Yeivin R., Sacks T. G. Pathogenesis of Escherichia coli enteritis in the ligated rabbit gut. Isr J Med Sci. 1967 May-Jun;3(3):445–452. [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., Smith J. W., Miller T. E., Barnett J. A., Sanford J. P. Immunoglobulin synthesis in lower urinary tract infection. J Lab Clin Med. 1970 Jan;75(1):19–29. [PubMed] [Google Scholar]
- Hinman F., Jr, Cox C. E. Residual urine volume in normal male subjects. J Urol. 1967 Apr;97(4):641–645. doi: 10.1016/S0022-5347(17)63092-1. [DOI] [PubMed] [Google Scholar]
- Hinman F., Jr, Cox C. E. The voiding vesical defense mechanism: the mathematical effect of residual urine, voiding interval and volume on bacteriuria. J Urol. 1966 Oct;96(4):491–498. doi: 10.1016/S0022-5347(17)63297-X. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh I. P., Hammond B. J., Watson B. W., O'Grady F. Theory of hydrokinetic clearance of bacteria from the urinary bladder. I. Effect of variations in bacterial growth rate. Invest Urol. 1975 May;12(6):468–472. [PubMed] [Google Scholar]
- Monis B., Dorfman H. D. Some histochemical observations on transitional epithelium of man. J Histochem Cytochem. 1967 Aug;15(8):475–481. doi: 10.1177/15.8.475. [DOI] [PubMed] [Google Scholar]
- Mulholland S. G., Foster E. A., Paquin A. J., Jr, Gillenwater J. Y. The effect of rabbit vesical mucosa on bacterial growth. Invest Urol. 1969 May;6(6):593–604. [PubMed] [Google Scholar]
- Norden C. W., Green G. M., Kass E. H. Antibacterial mechanisms of the urinary bladder. J Clin Invest. 1968 Dec;47(12):2689–2700. doi: 10.1172/JCI105952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. L., Greenspan C., Moore S. W., Mulholland S. G. Role of surface mucin in primary antibacterial defense of bladder. Urology. 1977 Jan;9(1):48–52. doi: 10.1016/0090-4295(77)90284-9. [DOI] [PubMed] [Google Scholar]
- Parsons C. L., Greenspan C., Mulholland S. G. The primary antibacterial defense mechanism of the bladder. Invest Urol. 1975 Jul;13(1):72–78. [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrom S. H., Parsons C. L., Mulholland S. G. Role of urothelial surface mucoprotein in intrinsic bladder defense. Urology. 1977 May;9(5):526–533. doi: 10.1016/0090-4295(77)90245-x. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Hand W. L. Immunoglobulin content and antibody activity in urine in experimental urinary tract infection. J Immunol. 1972 Apr;108(4):861–866. [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., King G., Zeligs B. Studies on gonococcus infection. VIII. 125Iodine labeling of gonococci and studies on their in vitro interactions with eukaryotic cells. Infect Immun. 1975 Mar;11(3):453–459. doi: 10.1128/iai.11.3.453-459.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehling D. T., Steihm E. R. Elevated urinary secretory IgA in children with urinary tract infection. Pediatrics. 1971 Jan;47(1):40–46. [PubMed] [Google Scholar]
- Uehling D. T., Wolf L. Enhancement of the bladder defense mechanism by immunization. Invest Urol. 1969 Mar;6(5):520–526. [PubMed] [Google Scholar]
- Waldman R. H., Ganguly R. Immunity to infections on secretory surfaces. J Infect Dis. 1974 Oct;130(4):419–440. doi: 10.1093/infdis/130.4.419. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. Adherence of Neisseria gonorrhoeae to urethral mucosal cells: an electron-microscopic study of human gonorrhea. J Infect Dis. 1972 Dec;126(6):601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Wernet P., Breu H., Knop J., Rowley D. Antibacterial action of specific IgA and transport of IgM, IgA, and IgG from serum into the small intestine. J Infect Dis. 1971 Aug;124(2):223–226. doi: 10.1093/infdis/124.2.223. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Hohmann A. W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974 Oct;10(4):776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


