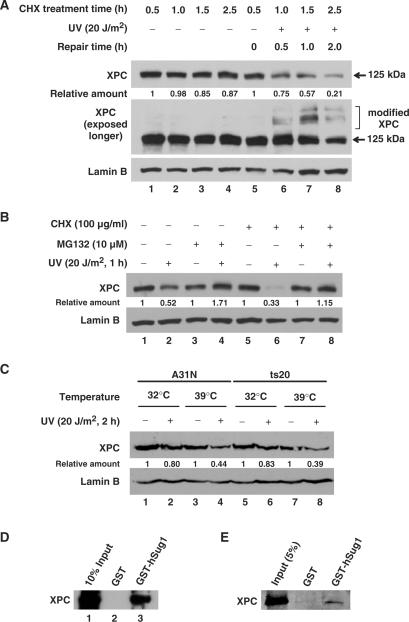
Figure 1.
XPC is degraded independent of ubiquitylation upon UV irradiation. (A) OSU-2 cells were treated with 100 μg/ml of CHX 0.5 h prior to UV irradiation at 20 J/m2 or mock treatment. Cells were further incubated in the medium containing CHX for different time periods. The whole cell lysates were subjected to western blot analysis using anti-XPC antibody. The same membrane was also immunoblotted for Lamin B as a loading control. (B) OSU-2 cells were treated with either MG132 (10 µM) or CHX (100ug/ml) or both for 1 h prior to UV irradiation at 20 J/m2 or mock treatment and the cells were incubated in the same medium for another 1 h. The cell lysates were subjected to immunoblotting as described above. (C) A31N and ts20 cells were cultured for 16 h at 32°C or 39°C, UV irradiated at 20 J/m2, and cultured for another 1 h at the same temperatures. The whole cell lysates were subjected to immunoblotting and the level of XPC was detected with anti-XPC antibody. (D and E) The cell lysates from OSU-2 cells (D) or purified recombinant XPC protein (E) were incubated with recombinant GST or GST-hSug1 proteins bound to GST beads. The protein bound to the beads was subjected to immunoblotting using anti-XPC antibody. Relative amount of total XPC at various times post-UV or mock-treatment were quantified relative to the respective unirradiated levels and normalized by Lamin B controls.