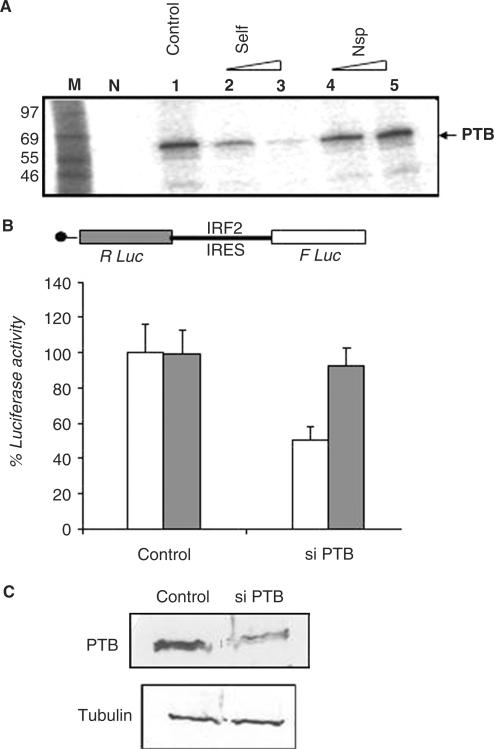
Figure 4.
Binding of PTB with the IRF2-IRES and effect of its silencing on the activity. (A) Purified recombinant PTB protein was incubated with 32P IRF2 5′UTR in absence (lane 1) or presence of 100-(lane 2) and 500-fold (lane 3) molar excess of self-cold IRF2 5′UTR (Lanes 2–3) or non-specific RNA (lanes 4–5). The RNA protein complexes were UV cross-linked and analyzed on SDS 10% PAGE followed by phosphorimaging. Lane M represents the molecular weight marker and lane N represents no protein control. (B) HeLa monolayer cells were transiently transfected with bicistronic plasmids pRIRF2F in absence or presence of 100 nM siPTB. Luciferase activity was measured 36 h post-transfection. FLuc (white bar) and RLuc (gray bar) activities are shown as fold decrease with respect to corresponding control (without siRNA), taken as 100%. The data mean ± SD from three independent experiments. (C) Western blot analysis of the above transfected cell lysates (40 μg), using anti-PTB antibody and anti α-tubulin antibody.