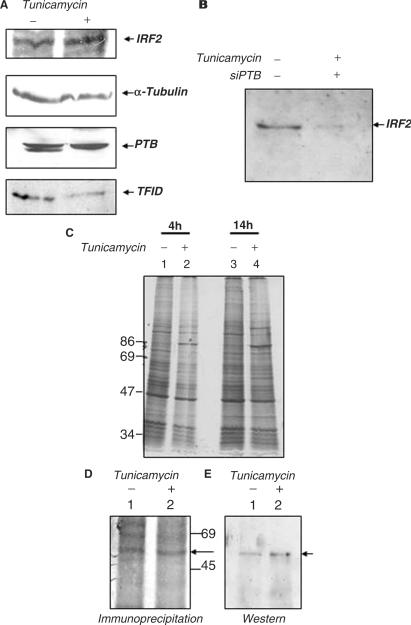
Figure 7.
Effect of tunicamycin and transient PTB silencing on IRF2 protein synthesis. (A) 100 μg of untreated and tunicamycin-treated HeLa extracts (as indicated above the panel) were probed with anti- IRF2, anti-α tubulin, anti-PTB and anti-TFIID antibodies. (B) HeLa cells were either mock-treated or treated with 100 nM siPTB. Six hours post siPTB transfection, 2.5 μg/ml of tunicamycin was added to the siPTB-treated cells as indicated above the panel. Extracts were prepared 14 h post tunicamycin treatment and western blot was performed with anti IRF2 antibody. (C) Metabolic labeling of untreated and tunicamycin-treated HeLa cells. HeLa cells were treated with 2.5 μg/ml tunicamycin for the indicated time points and metabolically labeled with 35S-methionine. The extracts were resolved in SDS–8% PAGE gel, followed by autoradiography. Lanes 1 and 3 indicate untreated extracts and lanes 2 and 4 depict tunicamycin-treated extracts. (D) Immunoprecipitation followed by western blot analysis of IRF2 in untreated and tunicamycin-treated extracts. Cells were metabolically labeled in the absence and presence of tunicamycin (as indicated above lanes). Lanes 1 and 2 correspond to immunoprecipitation of IRF2 without and with tunicamycin-treated cell extracts respectively, the arrow indicates the IRF2 protein band.(E) Lanes 1 and 2 correspond to the western blot of IRF2 with the above immunoprecipitated extracts.