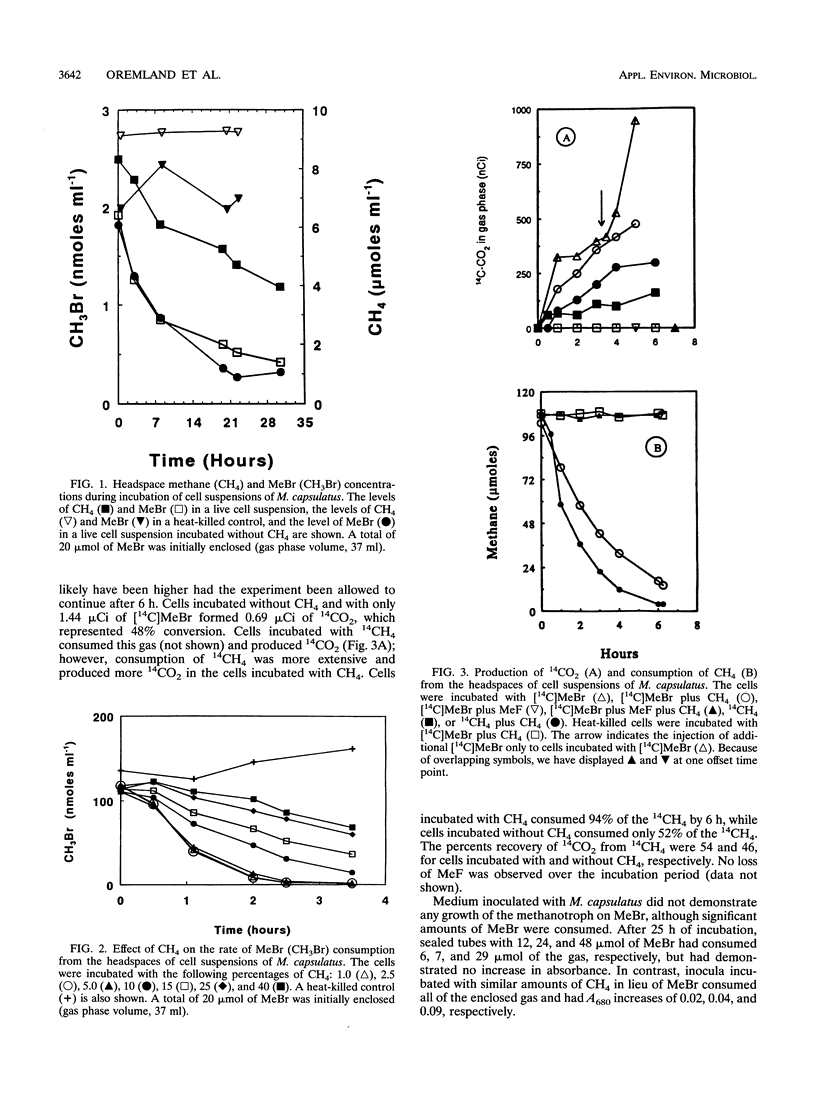
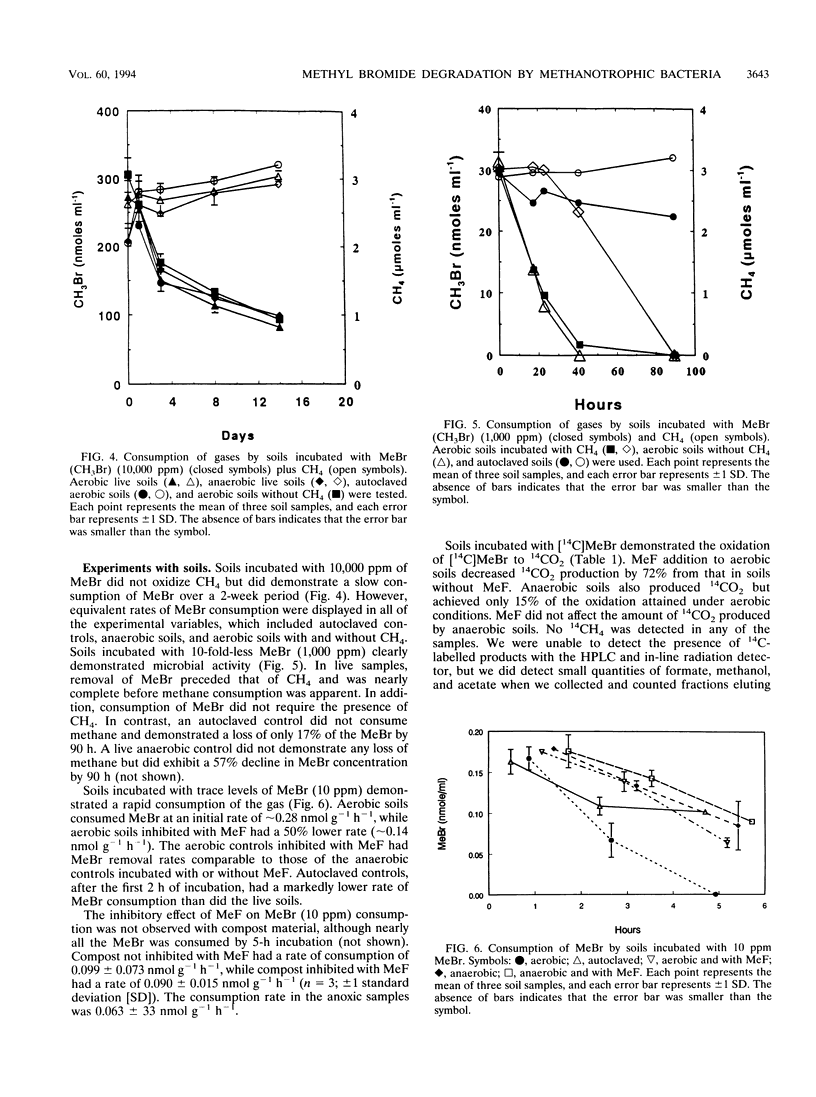
Abstract
Cell suspensions of Methylococcus capsulatus mineralized methyl bromide (MeBr), as evidence by its removal from the gas phase, the quantitative recovery of Br- in the spent medium, and the production of 14CO2 from [14C]MeBr. Methyl fluoride fluoride (MeF) inhibited oxidation of methane as well as that of [14C]MeBr. The rate of MeBr consumption by cells varied inversely with the supply of methane, which suggested a competitive relationship between these two substrates. However, MeBr did not support growth of the methanotroph. In soils exposed to high levels (10,000 ppm) of MeBr, methane oxidation was completely inhibited. At this concentration, MeBr removal rates were equivalent in killed and live controls, which indicated a chemical rather than biological removal reaction. At lower concentration (1,000 ppm) of MeBr, methanotrophs were active and MeBr consumption rates were 10-fold higher in live controls than in killed controls. Soils exposed to trace levels (10 ppm) of MeBr demonstrated complete consumption within 5 h of incubation, while controls inhibited with MeF or incubated without O2 had 50% lower removal rates. Aerobic soils oxidized [14C]MeBr to 14CO2, and MeF inhibited oxidation by 72%. Field experiments demonstrated slightly lower MeBr removal rates in chambers containing MeF than in chambers lacking MeF. Collectively, these results show that soil methanotrophic bacteria, as well as other microbes, can degrade MeBr present in the environment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby J., Dalton H., Whittenbury R. An improved assay for bacterial methane mono-oxygenase: some properties of the enzyme from Methylomonas methanica. Biochem J. 1975 Nov;151(2):459–462. doi: 10.1042/bj1510459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Strohmaier F. E., Oremland R. S. Acetylene as a substrate in the development of primordial bacterial communities. Orig Life Evol Biosph. 1988;18(4):397–407. doi: 10.1007/BF01808218. [DOI] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manö S., Andreae M. O. Emission of methyl bromide from biomass burning. Science. 1994 Mar 4;263(5151):1255–1257. doi: 10.1126/science.263.5151.1255. [DOI] [PubMed] [Google Scholar]
- Miller L. G., Coutlakis M. D., Oremland R. S., Ward B. B. Selective inhibition of ammonium oxidation and nitrification-linked n(2)o formation by methyl fluoride and dimethyl ether. Appl Environ Microbiol. 1993 Aug;59(8):2457–2464. doi: 10.1128/aem.59.8.2457-2464.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Culbertson C. W. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992 Sep;58(9):2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Hydrogen metabolism by decomposing cyanobacterial aggregates in big soda lake, nevada. Appl Environ Microbiol. 1983 May;45(5):1519–1525. doi: 10.1128/aem.45.5.1519-1525.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche Madeline E., Hyman Michael R., Arp Daniel J. Biodegradation of Halogenated Hydrocarbon Fumigants by Nitrifying Bacteria. Appl Environ Microbiol. 1990 Aug;56(8):2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K., Williams J., Wang N. Y., Cicerone R. J. Agricultural soil fumigation as a source of atmospheric methyl bromide. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8420–8423. doi: 10.1073/pnas.90.18.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]


